Which Group Tends To Form 1 Ions
Which Group Tends To Form 1 Ions - Whats the opposite of electron. Group 2 metals, the alkaline. Which group tends to not form ions or react? Which group on the periodic table tends to form 1+ ions? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. They have 1 valance electron to lose to become a noble gas.
Group 2 metals, the alkaline. Which group on the periodic table tends to form 1+ ions? Which group tends to not form ions or react? They have 1 valance electron to lose to become a noble gas. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Whats the opposite of electron.
They have 1 valance electron to lose to become a noble gas. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Group 2 metals, the alkaline. Whats the opposite of electron. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group on the periodic table tends to form 1+ ions? Which group tends to not form ions or react?
Is Oxygen a Positive or Negative Ion? Infrared for Health
Which group on the periodic table tends to form 1+ ions? Whats the opposite of electron. Group 2 metals, the alkaline. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized.
Question Video Connecting the Group Number with the Subscript in an
Group 2 metals, the alkaline. They have 1 valance electron to lose to become a noble gas. Whats the opposite of electron. Which group on the periodic table tends to form 1+ ions? Which group tends to not form ions or react?
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest
Which group on the periodic table tends to form 1+ ions? They have 1 valance electron to lose to become a noble gas. Whats the opposite of electron. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Group 2 metals, the alkaline.
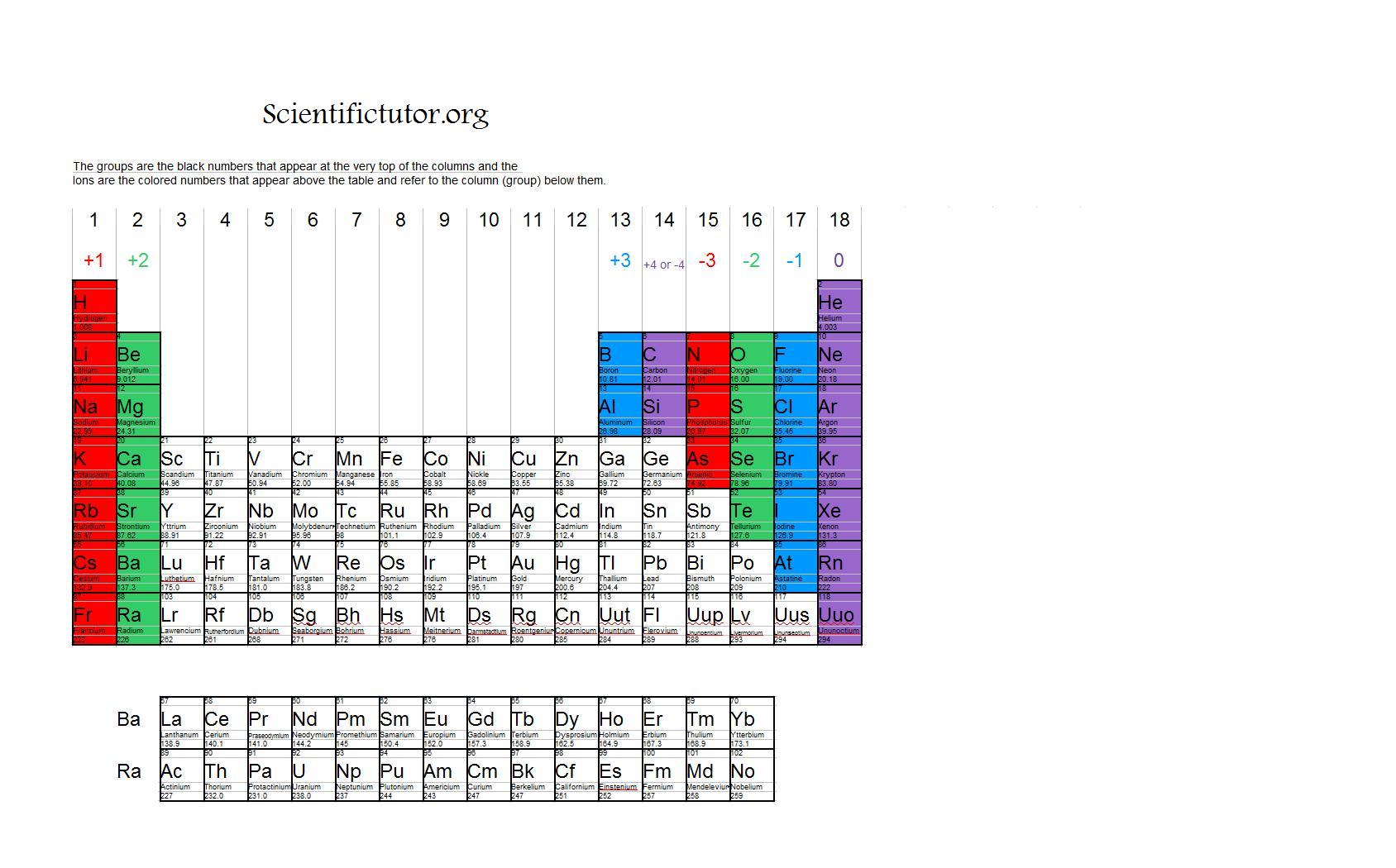
What Is The Charge Of Group 13 On Periodic Table
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Which group on the periodic table tends to form 1+ ions? Group 2 metals, the alkaline. Whats the opposite of electron. They have 1 valance electron to lose to become a noble gas.
Ionic Compound Nomenclature Presentation Chemistry
They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react? Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Whats the opposite of electron. Group 1 metals, the alkali metals, have the 1 valence.
Molecular and Ionic Compounds CHEM 1305 General Chemistry I—Lecture
Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group on the periodic table tends to form.
Solved Sort the following statements based on whether demand
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group on the periodic table tends to form 1+ ions? Group 2 metals, the alkaline. They have.
Metals and nonmetals The Basics Reactivity Reactions with
They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Whats the opposite of electron. Group 2 metals, the alkaline.
SOLVED ATOMIC RADIUS What trend in atomic radius do you see as you go
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Group 2 metals, the alkaline. They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react? Which group on the periodic table tends to form 1+ ions?
O Ion 19 39k+1 Possui
They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form.
Group 2 Metals, The Alkaline.
Which group on the periodic table tends to form 1+ ions? They have 1 valance electron to lose to become a noble gas. Whats the opposite of electron. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized.
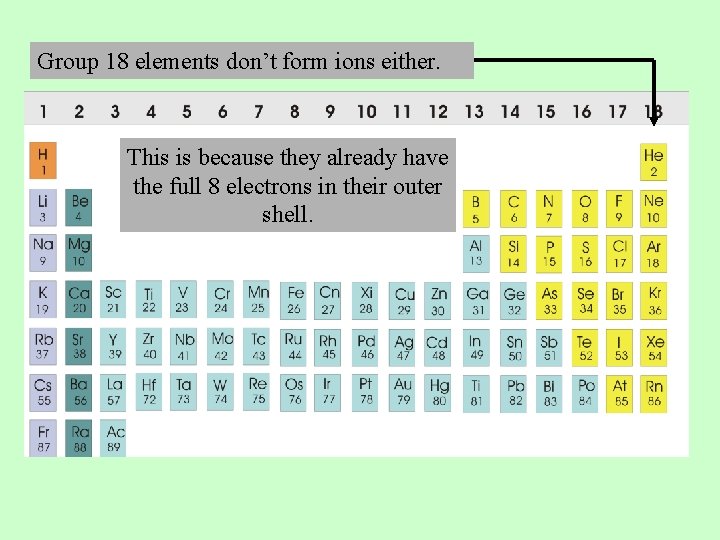
Which Group Tends To Not Form Ions Or React?
Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which.




.PNG)