When An Ionic Bond Forms Electrons Are
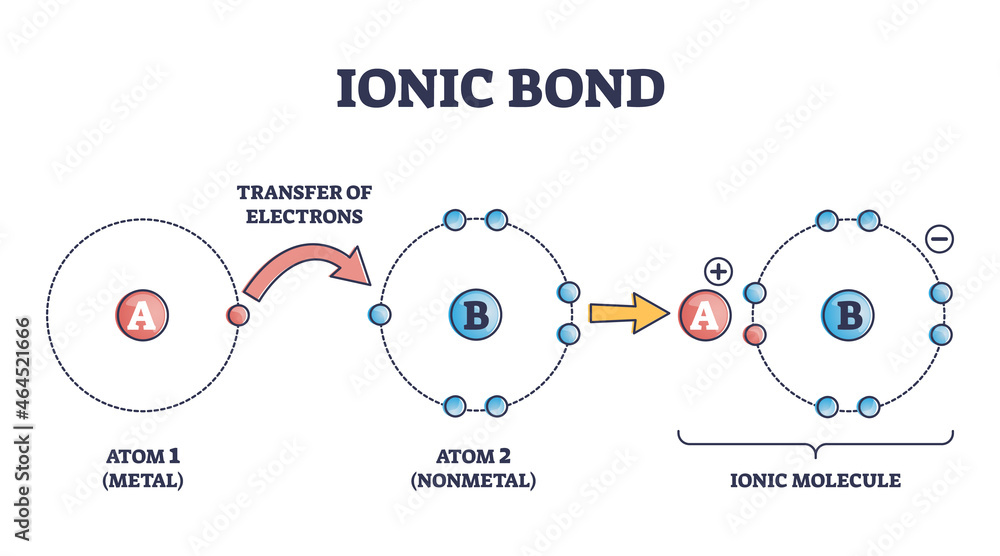
When An Ionic Bond Forms Electrons Are - What happens to electrons when an ionic bond forms? Ionic bonding is simply the electrostatic forces of attraction between ions,. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a.
A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. What happens to electrons when an ionic bond forms? Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their. Ionic bonding is simply the electrostatic forces of attraction between ions,.
A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Ionic bonding is simply the electrostatic forces of attraction between ions,. What happens to electrons when an ionic bond forms? Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their.
Ionic Bond Definition and Examples
Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Ionic bonding is simply the electrostatic forces of.
Ionic bonding CH 6.1 SC.912.P.8.4 SC.912.P ppt download
Ionic bonding is simply the electrostatic forces of attraction between ions,. What happens to electrons when an ionic bond forms? Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their. A cation (a positive ion) forms when a neutral atom loses one or more electrons from.
Ionic Bond Definition, Properties, Examples, Facts, 40 OFF
What happens to electrons when an ionic bond forms? Ionic bonding is simply the electrostatic forces of attraction between ions,. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Ionic bonding can result from a redox reaction when atoms of an.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
What happens to electrons when an ionic bond forms? Ionic bonding is simply the electrostatic forces of attraction between ions,. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Ionic bonding can result from a redox reaction when atoms of an.
Chemical Bonds Anatomy and Physiology I
Ionic bonding is simply the electrostatic forces of attraction between ions,. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give.
What Is An Ionic Bond Sciencing Ionic Bonding Ionic Chemical Bond
What happens to electrons when an ionic bond forms? A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Ionic bonding is simply the electrostatic forces of attraction between ions,. Ionic bonding can result from a redox reaction when atoms of an.
ionic bond Definition, Properties, Examples, & Facts Britannica
Ionic bonding is simply the electrostatic forces of attraction between ions,. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give.
Ionic bond and electrostatic attraction from chemical bonding outline
What happens to electrons when an ionic bond forms? Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when.
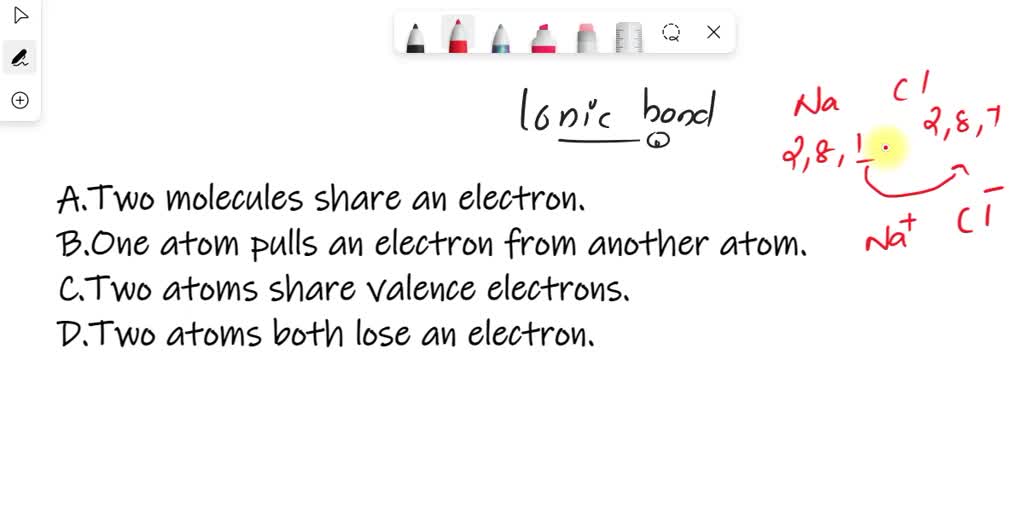
SOLVED Which best describes how an ionic bond forms? A. Two atoms
Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. What happens to electrons when an ionic bond.
ionic an bond forms when one atom gives up one or more electrons to
What happens to electrons when an ionic bond forms? Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when.
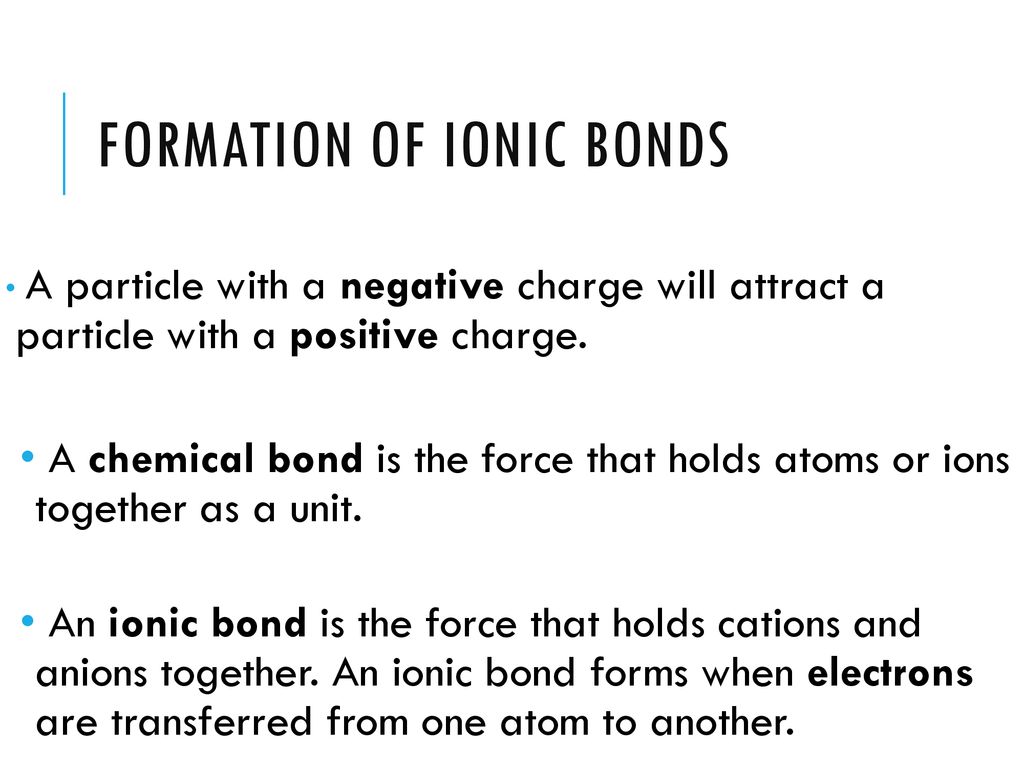
Ionic Bonding Can Result From A Redox Reaction When Atoms Of An Element (Usually Metal), Whose Ionization Energy Is Low, Give Some Of Their.
Ionic bonding is simply the electrostatic forces of attraction between ions,. What happens to electrons when an ionic bond forms? A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a.



.PNG)