What Is The Molecular Geometry Of Ozone O3
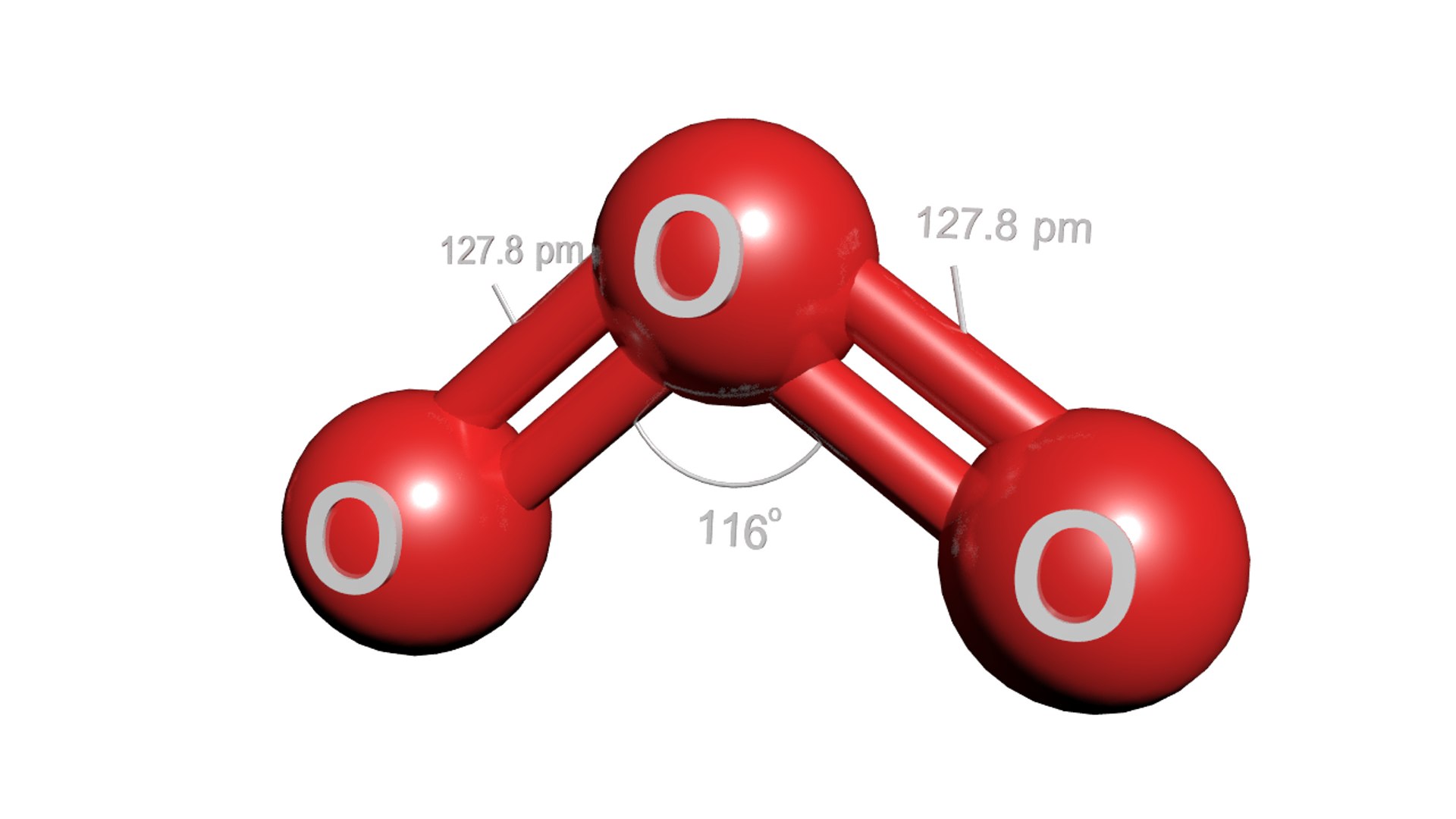
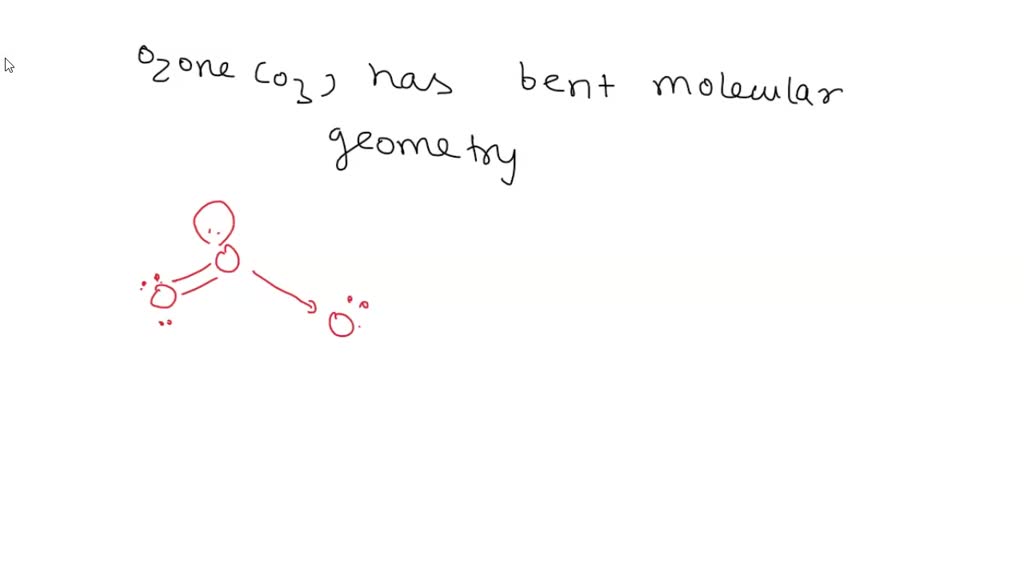
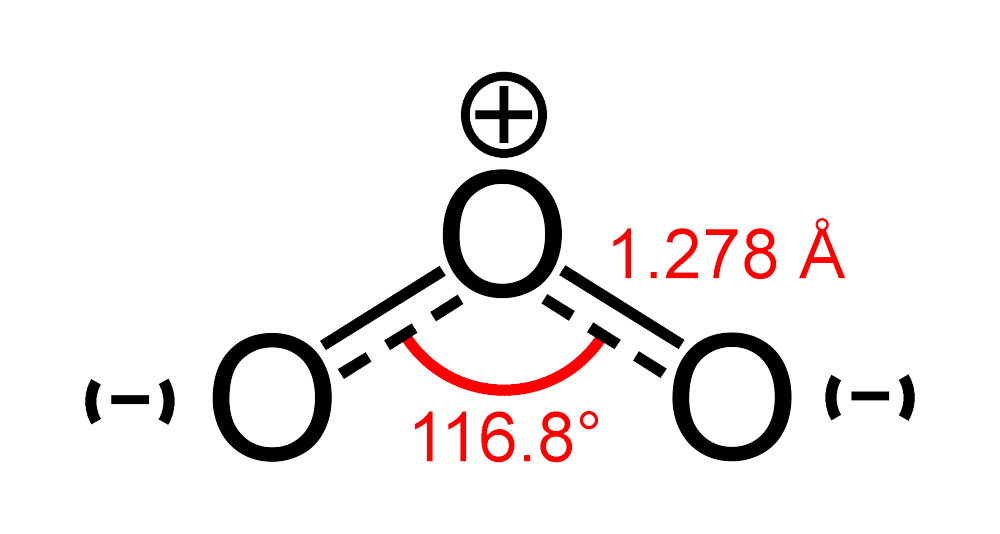
What Is The Molecular Geometry Of Ozone O3 - The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double boond and one single bond. Repulsion causes the bond angle to.
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance.
Repulsion causes the bond angle to. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. In the lewis structure of ozone, there are one double boond and one single bond.
3D O3 Molecule Ozone Model TurboSquid 1425810
In the lewis structure of ozone, there are one double boond and one single bond. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Ozone (o3).
O3 ozone molecule Royalty Free Vector Image VectorStock
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of.
SOLVED 1. Molecular shape of ozone (O3)? Molecular shape of ozone (O3
Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron.
What is O3 lewis structure and how to calculate the formal charge on it
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though.
3D o3 molecule ozone model TurboSquid 1425810
The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. In the lewis structure of ozone, there are one double boond and one single bond. Repulsion causes.
O3 Molecular Geometry,Shape and Bond Angles(Ozone) Molecular geometry
Repulsion causes the bond angle to. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of.
Which of the following is the correct molecular geometry for ozone. O3
The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair.
Ozone O3 Molecule Stock Photo Alamy
The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Repulsion causes the bond angle.
Is O3 Polar or Nonpolar? Techiescientist
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. In the lewis structure of ozone, there are one double boond and one single bond. Repulsion causes the bond angle to. The.
What is the \\angle OOO bond angle in ozone?
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Repulsion causes the bond angle to. The ozone molecule is found to be bent trigonal planar shape due to the presence of.
In The Lewis Structure Of Ozone, There Are One Double Boond And One Single Bond.
The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Repulsion causes the bond angle to.