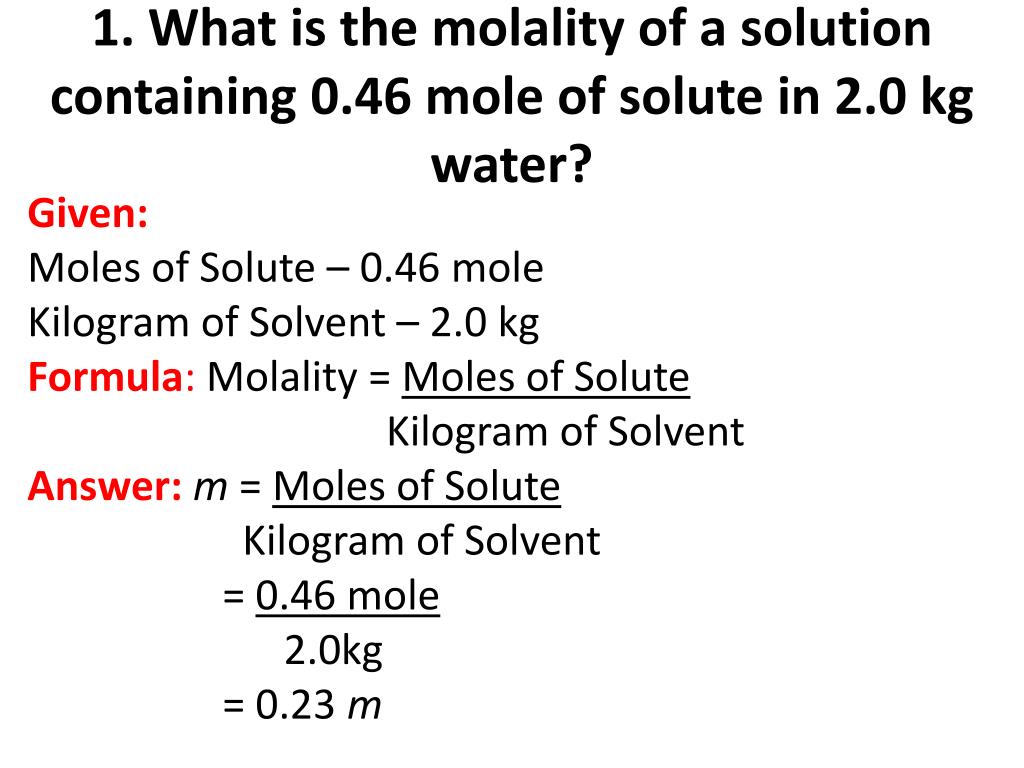
What Is The Molality Of A Solution Apex
What Is The Molality Of A Solution Apex - The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. A model that shows all bonds and atoms connected in the molecule. Mole 6.02 × 10^23 units of something,. Study with quizlet and memorize flashcards containing terms like avogadro's. Molality the concentration of a solution.
The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. A model that shows all bonds and atoms connected in the molecule. Molality the concentration of a solution. Mole 6.02 × 10^23 units of something,. Study with quizlet and memorize flashcards containing terms like avogadro's.
Study with quizlet and memorize flashcards containing terms like avogadro's. The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. A model that shows all bonds and atoms connected in the molecule. Molality the concentration of a solution. Mole 6.02 × 10^23 units of something,.
calculate the molality of a solution containing 20.7g of K2CO3
A model that shows all bonds and atoms connected in the molecule. Study with quizlet and memorize flashcards containing terms like avogadro's. The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. Molality the concentration of a solution. Mole 6.02 × 10^23 units of something,.
MahtaKaelah
The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. Molality the concentration of a solution. A model that shows all bonds and atoms connected in the molecule. Mole 6.02 × 10^23 units of something,.
SOLUTION Pharmaceutical Quantitative analysis moles, molality
The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. Mole 6.02 × 10^23 units of something,. A model that shows all bonds and atoms connected in the molecule. Study with quizlet and memorize flashcards containing terms.
Density of a 2.05 M solution of acetic acid in water is 1.02 g/ml. The
Molality the concentration of a solution. Study with quizlet and memorize flashcards containing terms like avogadro's. Mole 6.02 × 10^23 units of something,. A model that shows all bonds and atoms connected in the molecule. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution.
9. Differentiate between molarity and molality of a solution. How can we
The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. Molality the concentration of a solution. A model that shows all bonds and atoms connected in the molecule. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. Study with quizlet and memorize flashcards containing terms like.
Molality Example 1 ( Video ) Chemistry CK12 Foundation
Molality the concentration of a solution. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. A model that shows all bonds and atoms connected in the molecule. Mole 6.02 × 10^23 units of something,. The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution.
SOLUTION Molality png Studypool
The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. A model that shows all bonds and atoms connected in the molecule. Study with quizlet and memorize flashcards containing terms like avogadro's. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. Molality the concentration of a.
Molality of an aqueous solution of urea in which moke fraction of urea
The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. Molality the concentration of a solution. Mole 6.02 × 10^23 units of something,. The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. Study with quizlet and memorize flashcards containing terms like avogadro's.
Differentiate between molarity and molality for a solution. How does a
Mole 6.02 × 10^23 units of something,. A model that shows all bonds and atoms connected in the molecule. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. Molality the concentration of a solution. Study with quizlet and memorize flashcards containing terms like avogadro's.
Molarity, Molality, Volume & Mass Percent,Mole Fraction & Density
The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. A model that shows all bonds and atoms connected in the molecule. Study with quizlet and memorize flashcards containing terms like avogadro's. Mole 6.02 × 10^23 units of something,. The change in enthalpy occurring when solvent molecules surround solute molecules forming.
A Model That Shows All Bonds And Atoms Connected In The Molecule.
The change in enthalpy occurring when solvent molecules surround solute molecules forming a solution. The concentration of a solution expressed as the number of moles of solute dissolved in 1 l of solution. Mole 6.02 × 10^23 units of something,. Study with quizlet and memorize flashcards containing terms like avogadro's.