What Is The Hybridization Of The Central Atom In Sf6
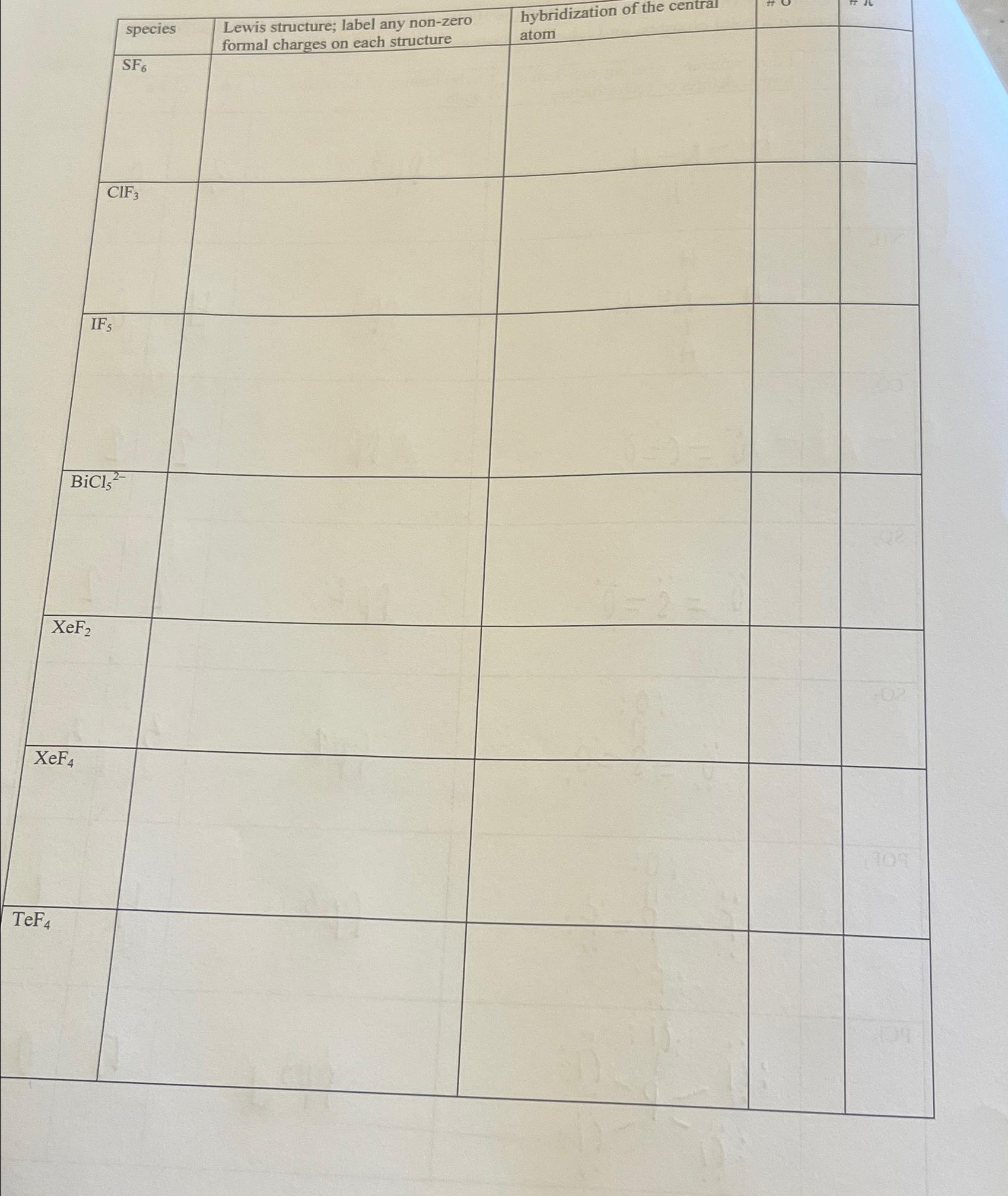
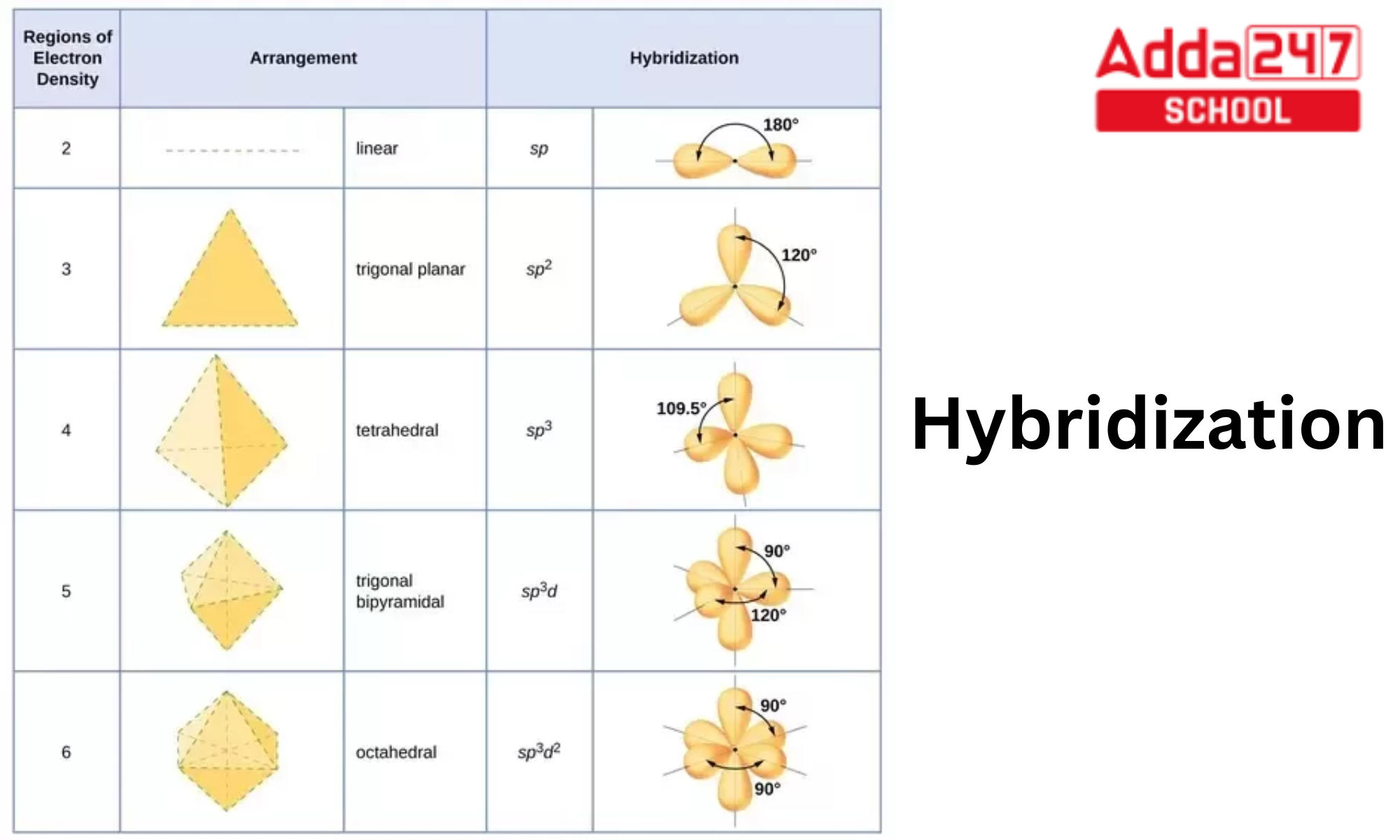
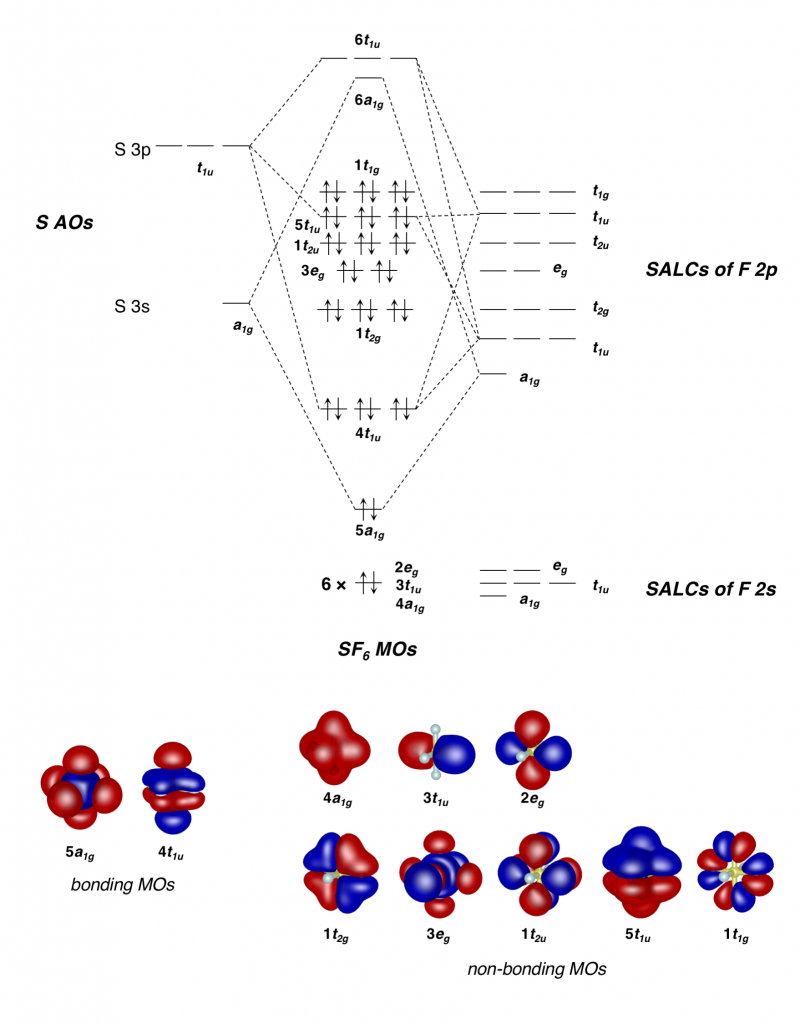
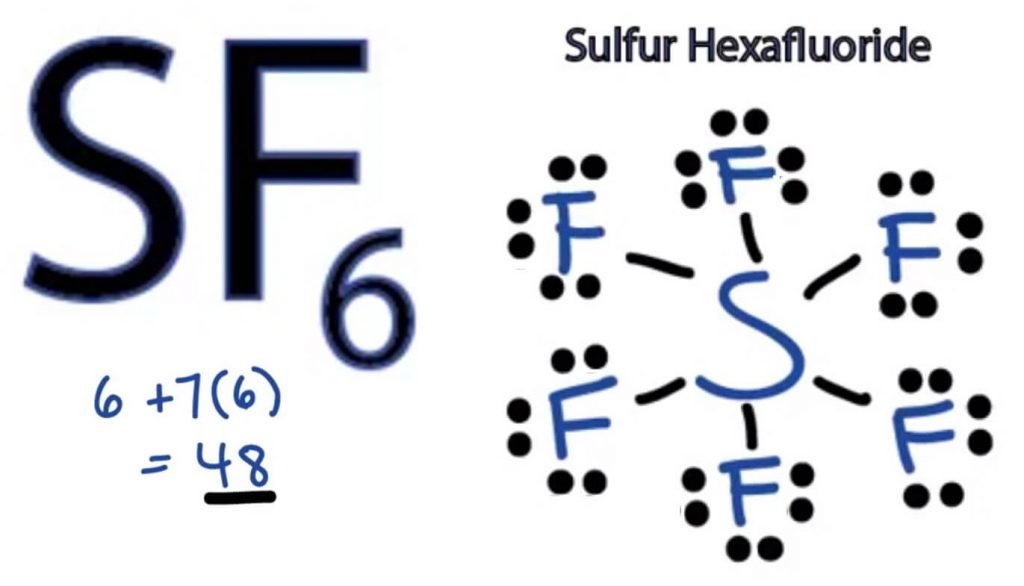
What Is The Hybridization Of The Central Atom In Sf6 - The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. A molecule of sulfur hexafluoride. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization.
A molecule of sulfur hexafluoride. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during.
A molecule of sulfur hexafluoride. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during.
Solved sf6 lewis structure hybridization of central atom
The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. A molecule of sulfur hexafluoride. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. In s f 6 the central sulphur atom has the ground state configuration,.
Hybridization and Hybrid Orbitals ChemTalk
The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. A molecule of sulfur hexafluoride. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. In s f 6 the central sulphur atom.
sp, sp2, sp3 Hybridization Examples, sp3d2 Shape & Structure
In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created.
What is the hybridization of the central atom in \ce{SF6}? Quizlet
Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds.
SF6 Lewis结构,分子几何,杂交,和MO图技术科学家万博网页版 万博网页版,万博体育app手机版登录
The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The orbitals involved in sulphur hexafluoride hybridization, as well as.
Hybridization of SF6 Molecule
The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The electronic configuration of sulfur is 1s 2 2s 2 2p 6.
Ceo 2024 Sf6 Hybridization Kally Mahala
Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. A molecule of sulfur hexafluoride. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The electronic.
SF6 Lewis structure, Molecular geometry, Bond angle, hybridization
The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The orbitals involved in sulphur hexafluoride hybridization, as well as the.
La geometría molecular SF6, la Estructura, la Forma y la polaridad de
In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. Now that we know the lewis structure of sf6, we can now.
Solved What is the hybridization of the central atom in SF6?
The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. A molecule of sulfur hexafluoride. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The sulfur atom in sulfur hexafluoride,.
The Sulfur Atom In Sulfur Hexafluoride, [Latex]\Ce{Sf6}[/Latex], Exhibits Sp 3 D 2 Hybridization.
The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d.
A Molecule Of Sulfur Hexafluoride.
Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule.