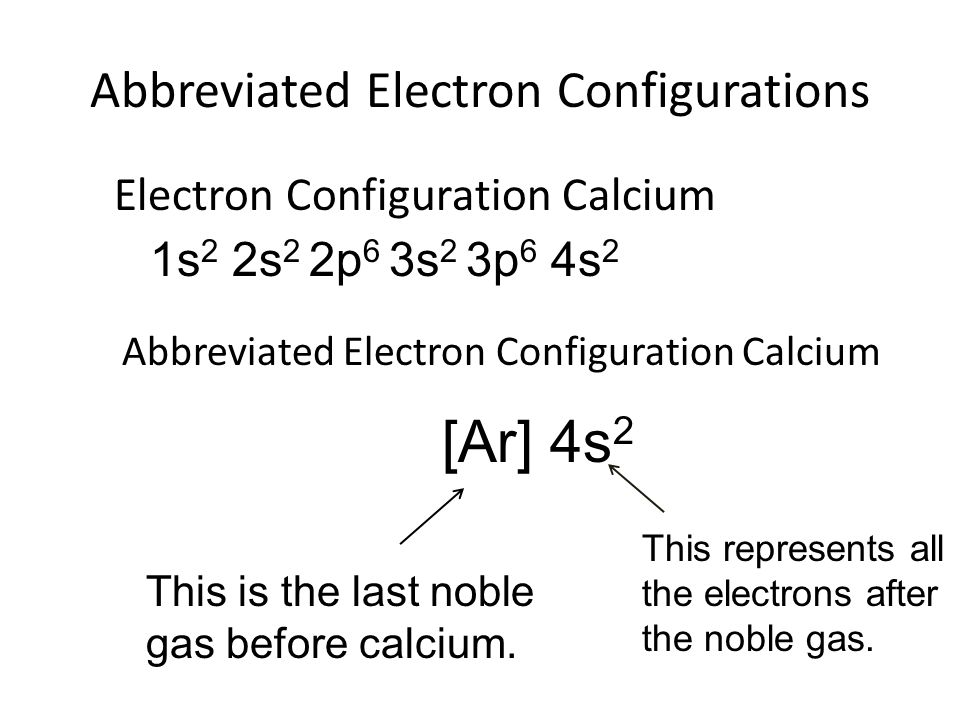
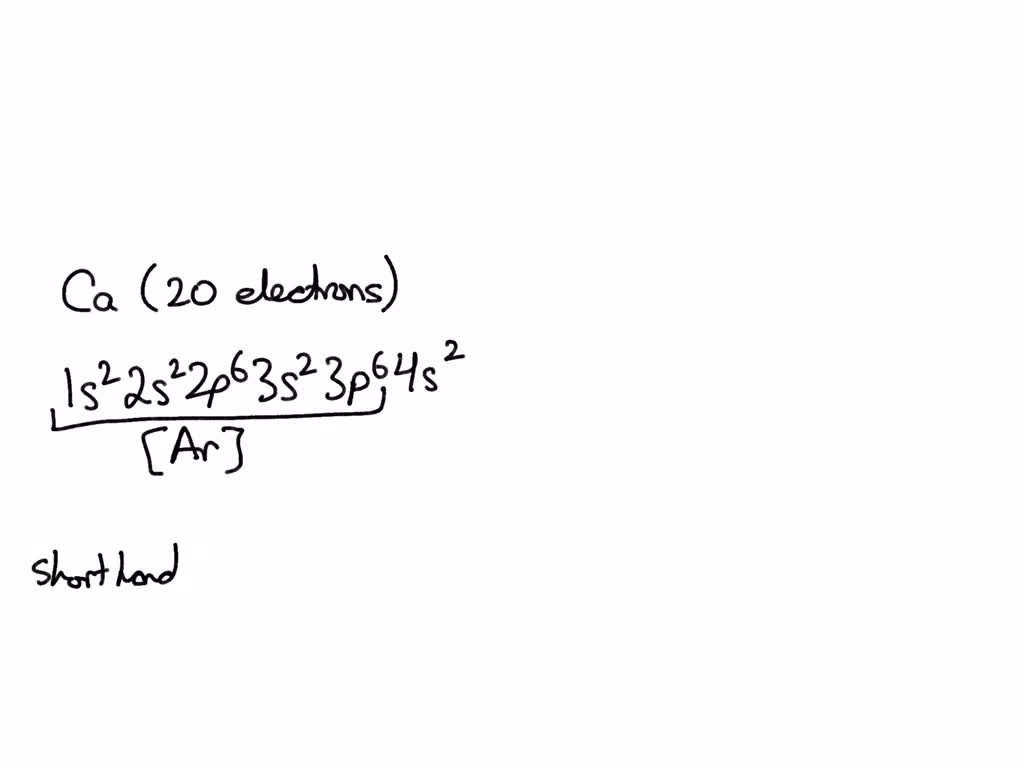What Is The Electron Configuration For Ca
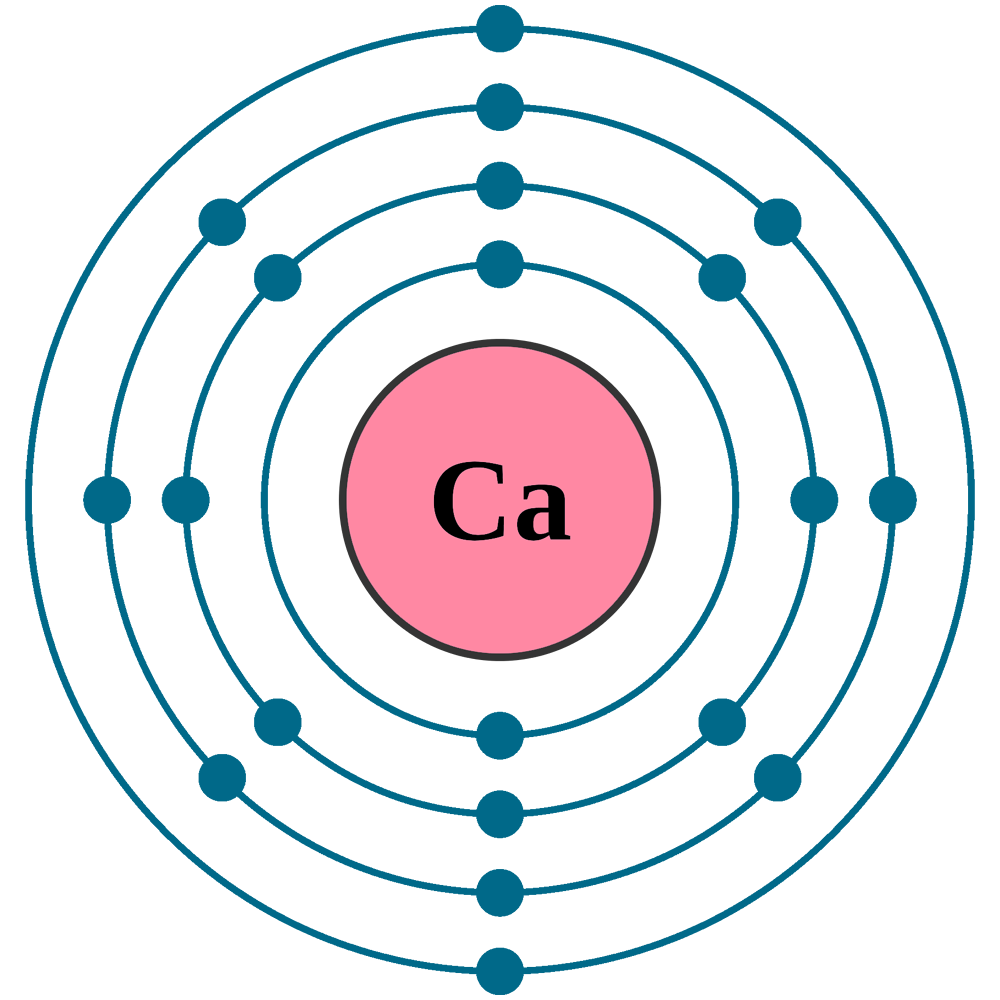
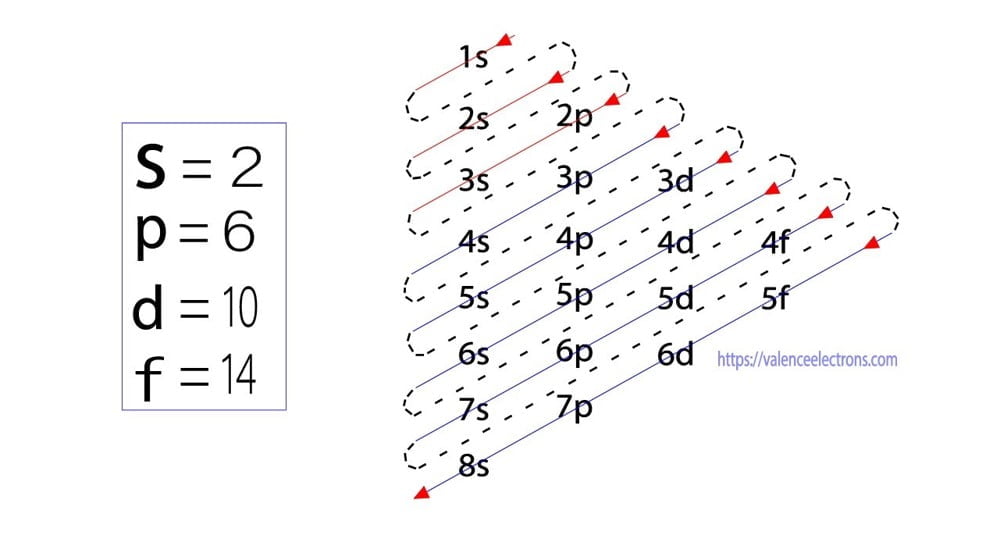
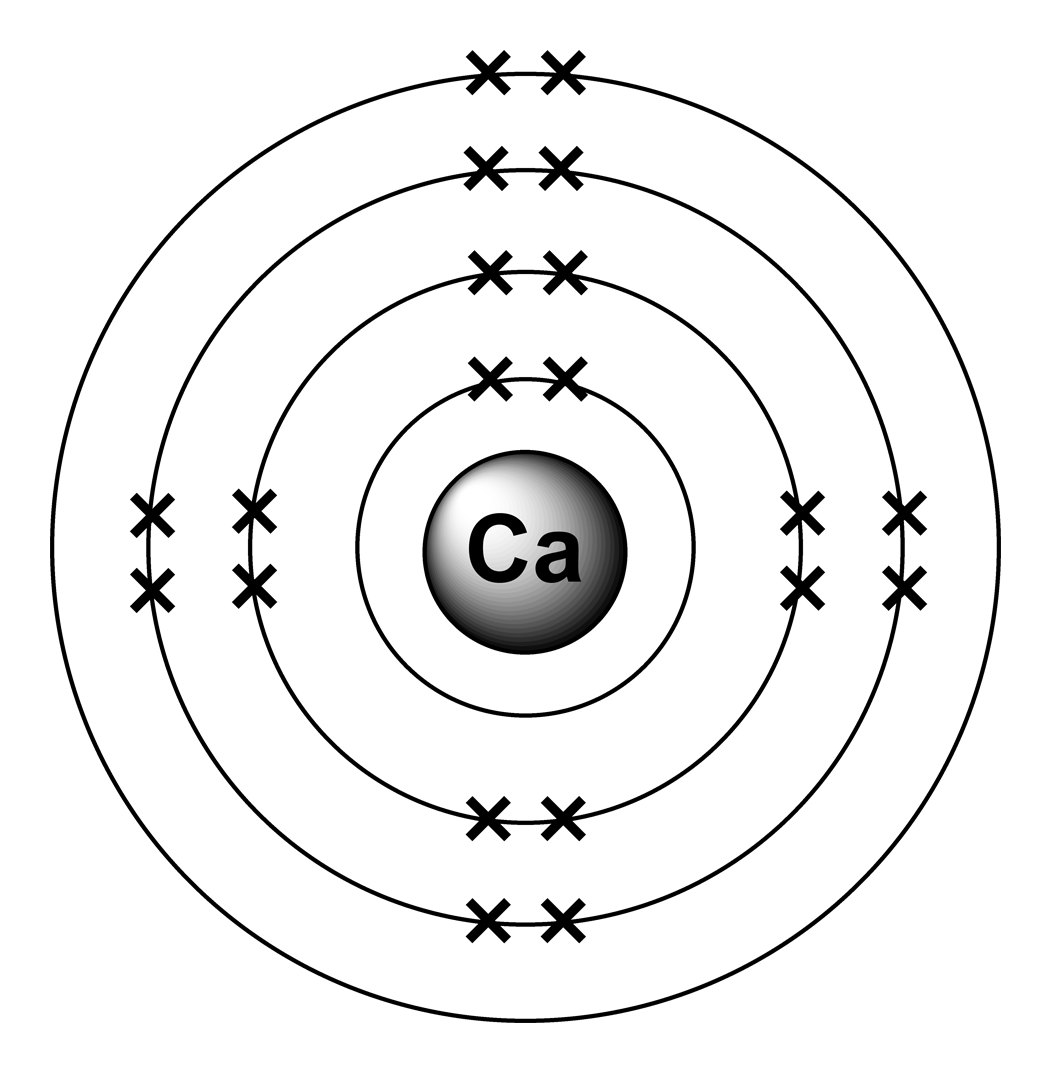
What Is The Electron Configuration For Ca - Since we need to take away two electrons, we first remove electrons from the. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). The shorthand electron configuration (or noble gas. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Electron configuration chart of all elements is mentioned in the table below. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6.
The shorthand electron configuration (or noble gas. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Since we need to take away two electrons, we first remove electrons from the. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Electron configuration chart of all elements is mentioned in the table below.
Since we need to take away two electrons, we first remove electrons from the. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas.
Calcium Ca (Element 20) of Periodic Table Elements FlashCards
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. The shorthand electron configuration (or noble gas. Since we need to take away two electrons, we first remove electrons from the. Electron configuration chart of all elements.
How to Write the Electron Configuration for Calcium (Ca)
Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Since we need to take away two electrons, we first remove electrons from the. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. The electron configuration of sodium is \(1s^2 2s^2 2p^6.
Calcium Electron Configuration YouTube
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Since we need to take away two electrons, we first remove electrons from the. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. In order to write the calcium electron configuration we first need to.
ca orbital diagram TravisMatteo
Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: The shorthand electron configuration (or noble gas. Since we need to take away two electrons, we first.
SOLVED Write the condensed (noblegas) electron configuration of Ca.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). Electron configuration chart of all elements is mentioned in the table below. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784.
Calcium electronic configuration How to Write Calcium electronic
Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Since we need to take away two.
Electron Configuration for Calcium (Ca, Ca2+ ion)
Electron configuration chart of all elements is mentioned in the table below. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). In order to write the calcium electron configuration we first need to know the number of electrons for the ca.
Electron arrangements
Electron configuration chart of all elements is mentioned in the table below. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: The shorthand electron configuration (or noble gas. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Hence,.
Calcium electron configuration Stock Image C029/5027 Science
Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. Since we need to take away two electrons, we first remove electrons from the..
Calcium Electron Configuration (Ca) with Orbital Diagram
Since we need to take away two electrons, we first remove electrons from the. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). 1s 2 2s 2 2p 6 3s 2.
Since We Need To Take Away Two Electrons, We First Remove Electrons From The.
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons).
Calcium Is A Chemical Element Of The Periodic Table With Chemical Symbol Ca And Atomic Number 20 With An Atomic Weight Of 40.0784 U And Is Classed As.
Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: