What Are Repelled In The Vsepr Theory
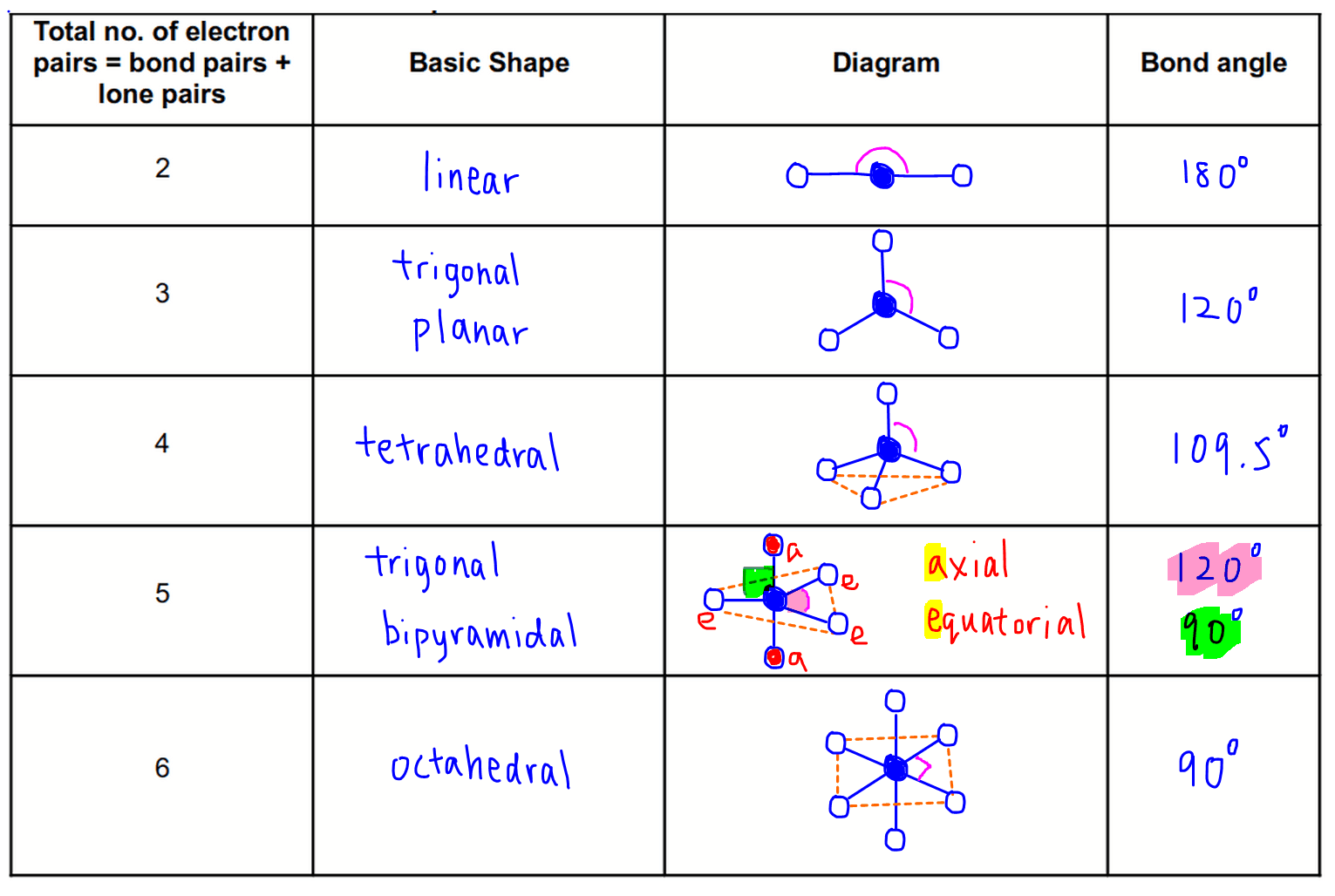
What Are Repelled In The Vsepr Theory - Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. It basically says that electron pairs,. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr).
The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. It basically says that electron pairs,. Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence.
Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). It basically says that electron pairs,.
VSEPR theory VSEPR सिद्धांत valency shell electron pair repulsion
In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. It basically says that electron pairs,. Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. The basic idea in molecular shapes is called.
VSEPR Theory Class 11 VSEPR Theory VSEPR Theory Bsc 1st Year
It basically says that electron pairs,. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone.
VSEPR THEORY’S BASIC ASSUMPTION & MAIN POSTULATES EXPLAINED WITH
Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. It basically says that electron pairs,. The basic idea in molecular shapes is called.
vsepr theory class11vsepr theory and molecular shapes
Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. It basically says that electron pairs,. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone.
VSEPR Theory II YouTube
It basically says that electron pairs,. Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. The basic idea in molecular shapes is called.
VSEPR Theory Explanation, Chart, and Examples
The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom..
VSEPR Theory YouTube
The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. It basically says that electron pairs,. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone.
Vsepr theory chart
It basically says that electron pairs,. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. The basic idea in molecular shapes is called.
VSEPR THEORY Assumptions of VSEPR Theory APPLICATIONS OF VSEPR
It basically says that electron pairs,. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone.
VSEPR Theory Postulates, Prediction of geometrical shapes, Limitations
Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr)..
It Basically Says That Electron Pairs,.
Valence shell electron pair repulsion theory (vsepr) states that the shapes of covalent mole cules depend on the fact that pairs of valence. In water (h₂o), vsepr theory indicates that there are two bonding pairs of electrons and two lone pairs around the central oxygen atom. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr).