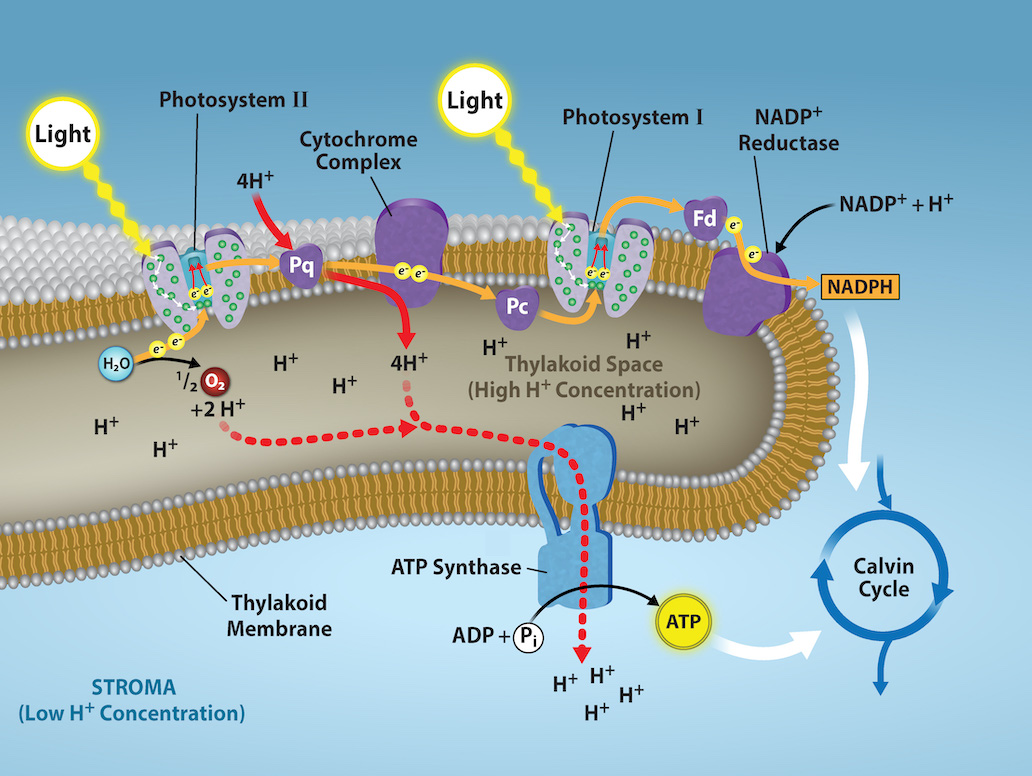
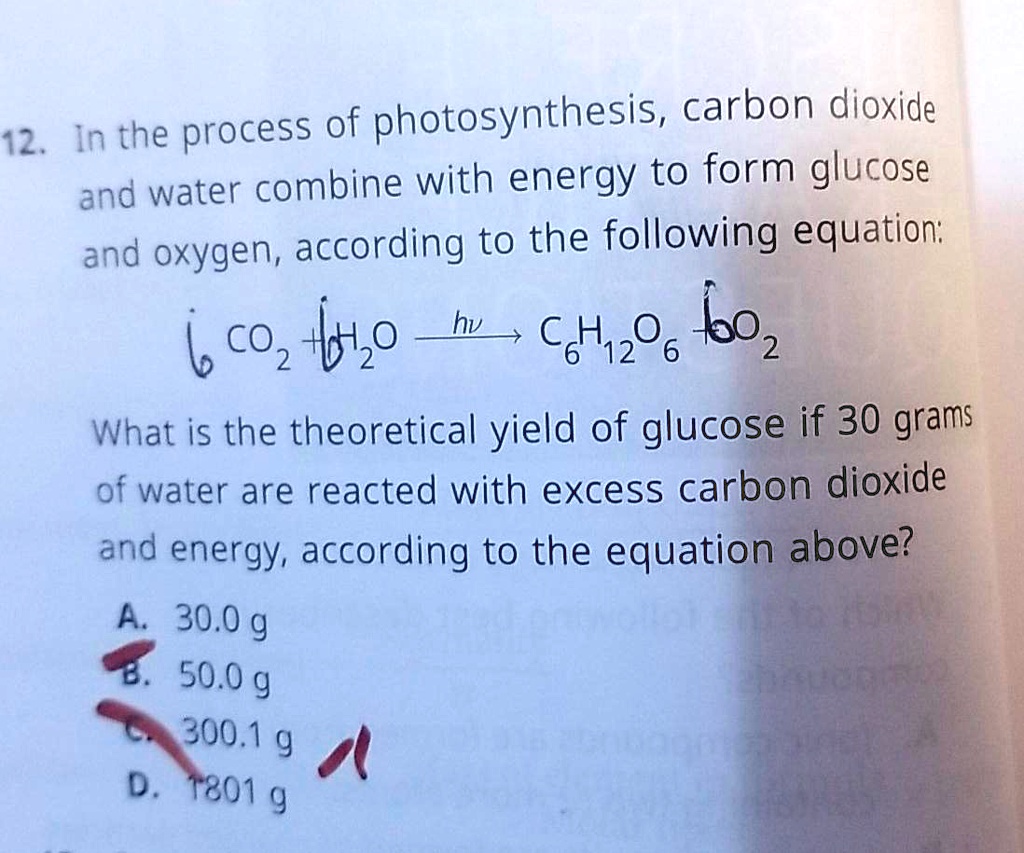Water And Carbon Dioxide Combine To Form
Water And Carbon Dioxide Combine To Form - Carbon dioxide reacts with ocean water. As carbon dioxide is present in the air is blown into the flask containing an. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere. The cloudy white solution observed when co 2 is. Carbon dioxide dissolves in water and slowly reacts with water to produce carbonic acid. When carbon dioxide mixes with seawater it has the effect of reducing the availability of carbonate ions, which many marine organisms—corals,. When carbon dioxide reacts with water a weak acid is formed.
When carbon dioxide mixes with seawater it has the effect of reducing the availability of carbonate ions, which many marine organisms—corals,. The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. As carbon dioxide is present in the air is blown into the flask containing an. When carbon dioxide reacts with water a weak acid is formed. Carbon dioxide reacts with ocean water. Carbon dioxide dissolves in water and slowly reacts with water to produce carbonic acid. The cloudy white solution observed when co 2 is.
Carbon dioxide dissolves in water and slowly reacts with water to produce carbonic acid. When carbon dioxide reacts with water a weak acid is formed. As carbon dioxide is present in the air is blown into the flask containing an. When carbon dioxide mixes with seawater it has the effect of reducing the availability of carbonate ions, which many marine organisms—corals,. The cloudy white solution observed when co 2 is. The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. Carbon dioxide reacts with ocean water.
Water vapor and carbon dioxide combine, causing what is known as the
Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. When carbon dioxide mixes with seawater it has the effect of reducing the availability of carbonate ions, which many marine organisms—corals,. The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere..
CO2 + H2O (Carbon dioxide + Water) ข้อมูลทั้งหมดเกี่ยวกับh2co3 aq h2o
The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. As carbon dioxide is present in the air is blown into the flask containing an. Carbon dioxide dissolves in water and.
Water Analysis Dissolved Carbon Dioxide Advanced BioTech
Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. As carbon dioxide is present in the air is blown into the flask containing an. Carbon dioxide dissolves in water and slowly reacts with water to produce carbonic acid. The cloudy white solution observed when co.
Pop Quiz 13 Things to Know About Photosynthesis Britannica
As carbon dioxide is present in the air is blown into the flask containing an. Carbon dioxide reacts with ocean water. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. The cloudy white solution observed when co 2 is. When carbon dioxide mixes with seawater.
Glucose + Oxygen → Carbon dioxide + Water + Energy (as ATP) ppt download
The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere. Carbon dioxide reacts with ocean water. When carbon dioxide reacts with water a weak acid is formed. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. When carbon dioxide mixes.
Molecular Solids Co2
Carbon dioxide dissolves in water and slowly reacts with water to produce carbonic acid. When carbon dioxide reacts with water a weak acid is formed. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. When carbon dioxide mixes with seawater it has the effect of.
Simulations & Videos for Lesson 5.8 Can Gases Dissolve in Water
The cloudy white solution observed when co 2 is. When carbon dioxide reacts with water a weak acid is formed. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. Carbon dioxide reacts with ocean water. Carbon dioxide dissolves in water and slowly reacts with water.
Water Vapor and Carbon Dioxide Combine, Causing What is Known As the
Carbon dioxide reacts with ocean water. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere. The cloudy white solution observed when co 2 is. As carbon dioxide is present in.
SOLVED In the process of photosynthesis, carbon dioxide (CO2) and
When carbon dioxide mixes with seawater it has the effect of reducing the availability of carbonate ions, which many marine organisms—corals,. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere..
Biology's most important equation "carbon dioxide + water → glucose
The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere. The cloudy white solution observed when co 2 is. Carbon dioxide reacts with ocean water. Water and carbon dioxide combine to form carbonic acid (h 2 co 3), a weak acid that breaks (or “dissociates”) into hydrogen ions (h. When carbon dioxide reacts with water.
When Carbon Dioxide Mixes With Seawater It Has The Effect Of Reducing The Availability Of Carbonate Ions, Which Many Marine Organisms—Corals,.
When carbon dioxide reacts with water a weak acid is formed. The cloudy white solution observed when co 2 is. As carbon dioxide is present in the air is blown into the flask containing an. The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere.
Water And Carbon Dioxide Combine To Form Carbonic Acid (H 2 Co 3), A Weak Acid That Breaks (Or “Dissociates”) Into Hydrogen Ions (H.
Carbon dioxide reacts with ocean water. Carbon dioxide dissolves in water and slowly reacts with water to produce carbonic acid.



.jpg)