Uranium Electron Configuration Long Form
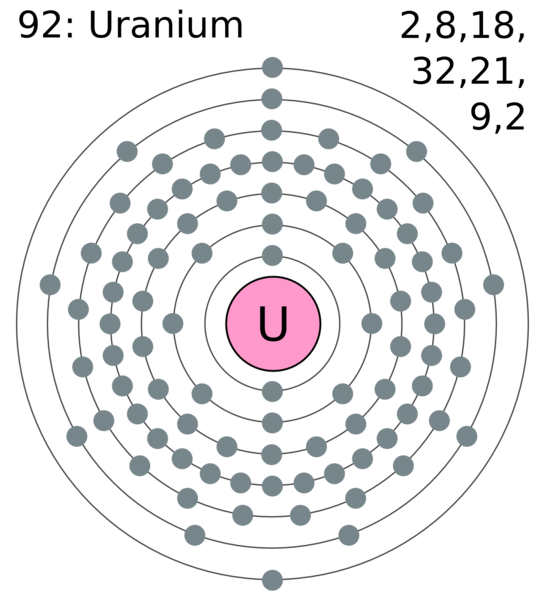
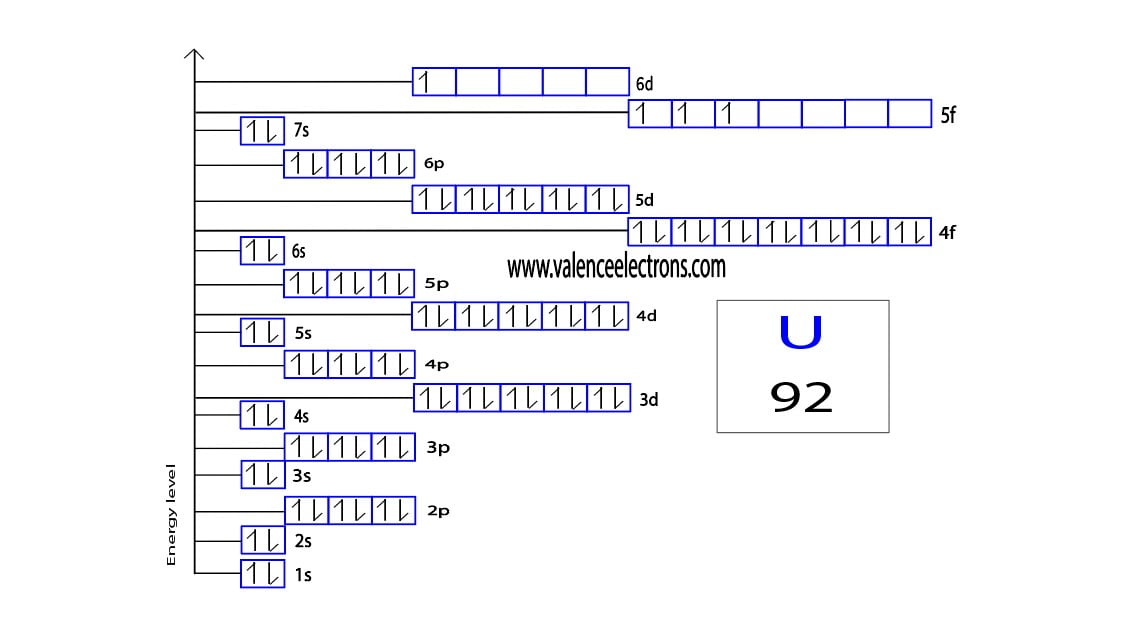
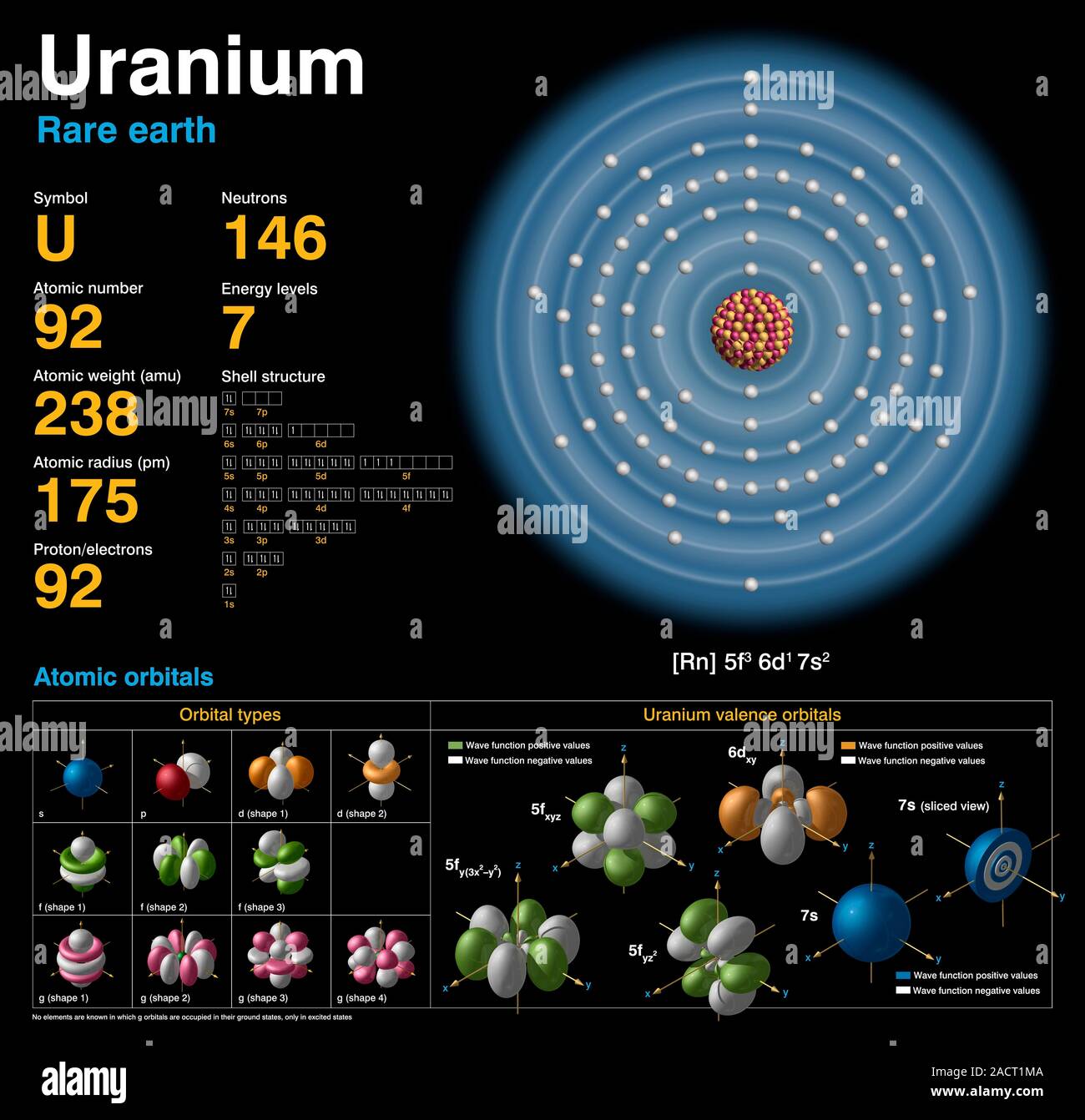
Uranium Electron Configuration Long Form - U (uranium) is an element with position number 92 in the periodic table. Electron configuration for uranium (element 92). Shell diagram of uranium (u) atom. Electron configuration provides information about the distribution of electrons. Atomic mass, electron configurations, charges, and more. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. Access detailed info on all elements: View rotating bohr models for all 118. The electron configuration of uranium is [rn] 5f3 6d1 7s2.
Electron configuration provides information about the distribution of electrons. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. View rotating bohr models for all 118. Access detailed info on all elements: The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. U (uranium) is an element with position number 92 in the periodic table. Shell diagram of uranium (u) atom. Atomic mass, electron configurations, charges, and more. The electron configuration of uranium is [rn] 5f3 6d1 7s2. The electron configuration for uranium (u) is based upon the placement of uranium in the fourth column of the actinide.
U (uranium) is an element with position number 92 in the periodic table. Electron configuration for uranium (element 92). Atomic mass, electron configurations, charges, and more. The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. Electron configuration provides information about the distribution of electrons. The electron configuration of uranium is [rn] 5f3 6d1 7s2. Access detailed info on all elements: Shell diagram of uranium (u) atom. The electron configuration for uranium (u) is based upon the placement of uranium in the fourth column of the actinide. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.
Lawrencium Electron Configuration
U (uranium) is an element with position number 92 in the periodic table. The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. Access detailed info on all elements: Electron configuration for uranium (element 92). View rotating bohr models for all 118.
Electron Configuration of Uranium U Lesson YouTube
The electron configuration for uranium (u) is based upon the placement of uranium in the fourth column of the actinide. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Access detailed info on all elements: U (uranium) is an element with position number 92 in the periodic table. View rotating bohr models for all.
Complete Electron Configuration for Uranium (U)
The electron configuration of uranium is [rn] 5f3 6d1 7s2. View rotating bohr models for all 118. Electron configuration for uranium (element 92). A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. U (uranium) is an element with position number 92 in the periodic table.
How To Find A Electron Configuration Of Uranium (U)
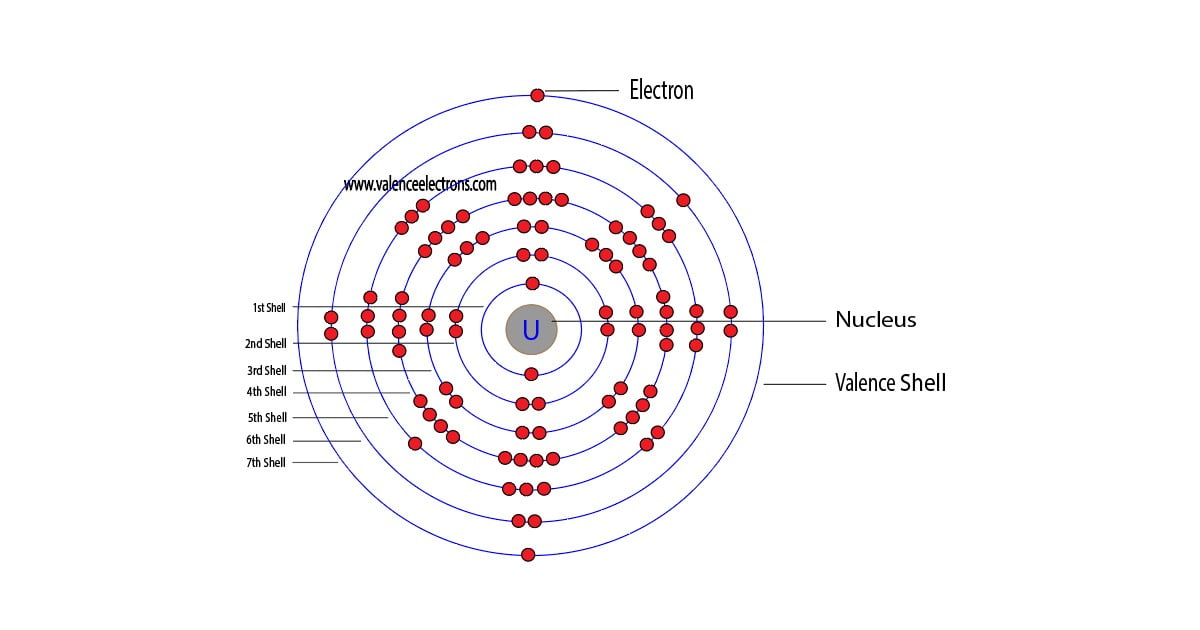
Shell diagram of uranium (u) atom. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. U (uranium) is an element with position number 92 in the periodic table. The electron configuration for uranium (u) is based upon the placement of uranium in the fourth column of the actinide. The electron configuration of uranium is.
24+ uranium orbital diagram KristineFrank
Electron configuration for uranium (element 92). View rotating bohr models for all 118. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Access detailed info on all elements: Shell diagram of uranium (u) atom.
How to Write the Electron Configuration for Uranium (U)
A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118. Electron configuration provides information about the distribution of electrons.
Electron Configuration of Plutonium Pu Lesson YouTube
U (uranium) is an element with position number 92 in the periodic table. Atomic mass, electron configurations, charges, and more. Shell diagram of uranium (u) atom. The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.
Uranium Protons Neutrons Électrons Configuration électronique
Electron configuration for uranium (element 92). The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. Access detailed info on all elements: Atomic mass, electron configurations, charges, and more. U (uranium) is an element with position number 92 in the periodic table.
Uranium (U). Diagram of the nuclear composition, electron configuration
The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals. View rotating bohr models for all 118. Access detailed info on all elements: Electron configuration for uranium (element 92). Atomic mass, electron configurations, charges, and more.
WebElements Periodic Table » Uranium » properties of free atoms
The electron configuration of uranium is [rn] 5f3 6d1 7s2. Electron configuration for uranium (element 92). Electron configuration provides information about the distribution of electrons. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals.
Access Detailed Info On All Elements:
The electron configuration for uranium (u) is based upon the placement of uranium in the fourth column of the actinide. Electron configuration for uranium (element 92). Shell diagram of uranium (u) atom. The electron configuration of uranium refers to the arrangement of electrons in the uranium atom’s orbitals.
View Rotating Bohr Models For All 118.
The electron configuration of uranium is [rn] 5f3 6d1 7s2. U (uranium) is an element with position number 92 in the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Electron configuration provides information about the distribution of electrons.