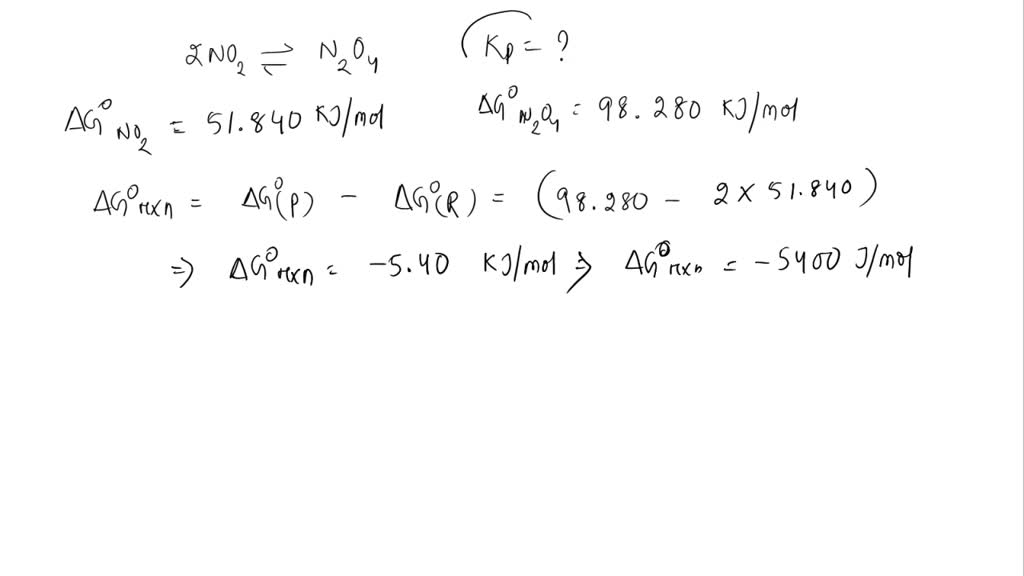Standard Molar Internal Energy Of Formation
Standard Molar Internal Energy Of Formation - We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the. How do you calculate standard molar enthalpy of formation? You use the standard enthalpy of the reaction and the enthalpies of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and.
You use the standard enthalpy of the reaction and the enthalpies of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. How do you calculate standard molar enthalpy of formation? We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the.
How do you calculate standard molar enthalpy of formation? We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. You use the standard enthalpy of the reaction and the enthalpies of.
(PDF) Standard molar Gibbs free energy of formation of PbO(s) over a
We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the. How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. You use the standard enthalpy of the.
Dimensionless standard molar Gibbs energies of formation of different
How do you calculate standard molar enthalpy of formation? You use the standard enthalpy of the reaction and the enthalpies of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for.
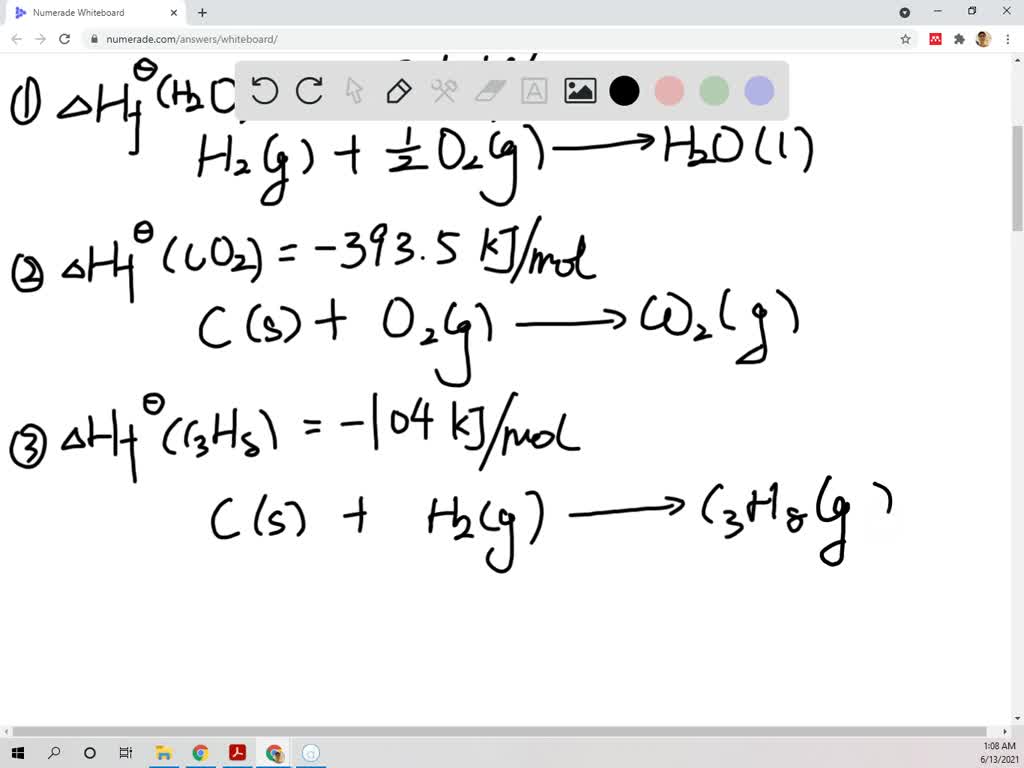
Using the standard molar enthalpies of formation give… SolvedLib
How do you calculate standard molar enthalpy of formation? You use the standard enthalpy of the reaction and the enthalpies of. We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of.
SOLVED The standard molar free energies of formation of NO2(g) and
You use the standard enthalpy of the reaction and the enthalpies of. We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Definition and explanation of.
[Solved] Calculate the standard molar entropy change for the formation
You use the standard enthalpy of the reaction and the enthalpies of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. How do you calculate standard molar.
The standard molar heat of formation of water is 258.8 kJ/mol.a. What
How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. You use the standard enthalpy of the reaction and the enthalpies of. We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar.
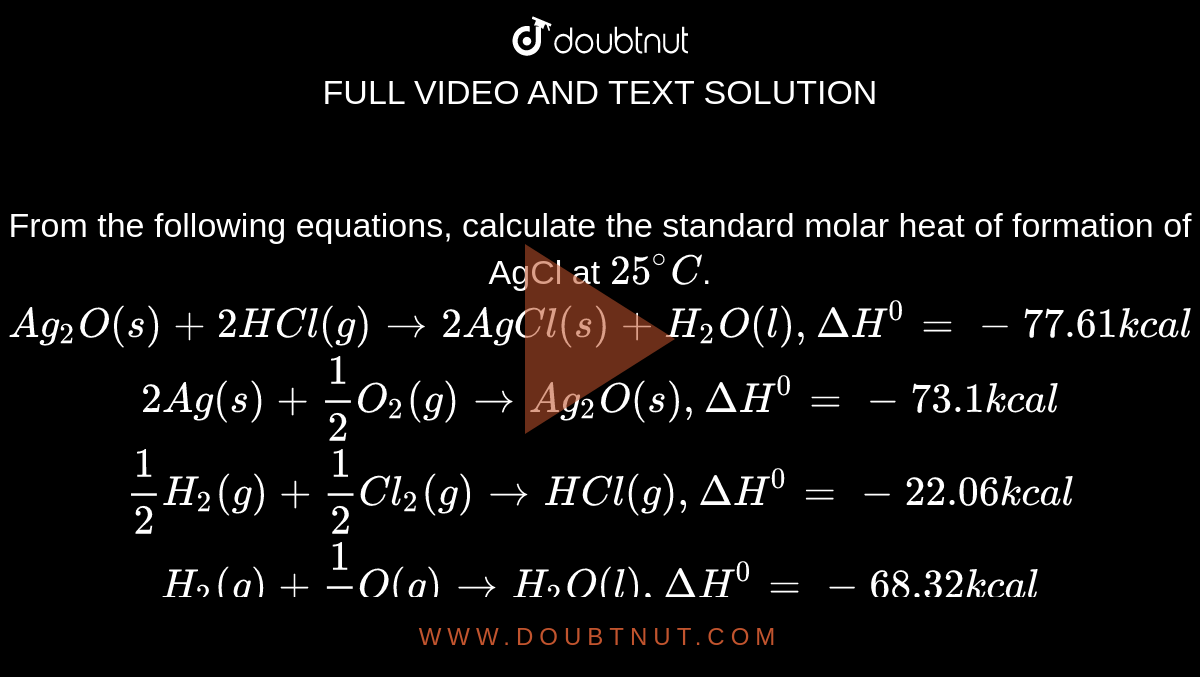
From the following equations, calculate the standard molar heat of
Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. You use the standard enthalpy of the reaction and the enthalpies of. How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for.
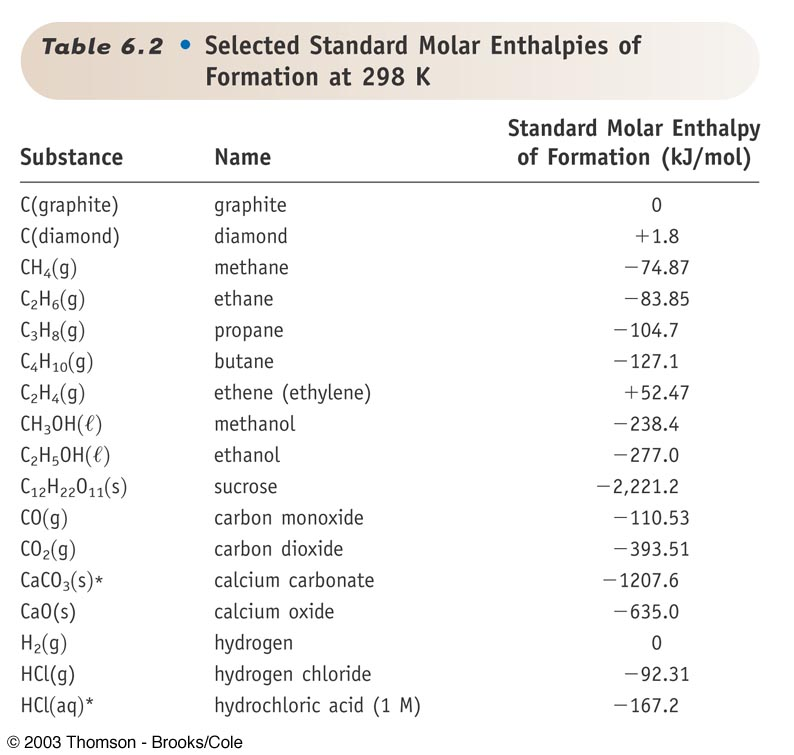
Solved Table 6.2. Selected Standard Molar Enthalpies of
We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the. You use the standard enthalpy of the reaction and the enthalpies of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. How do you calculate.
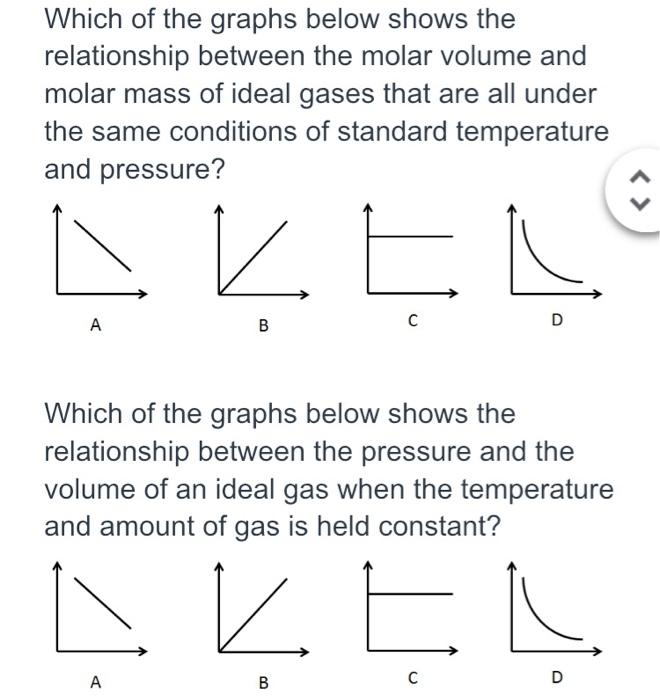
Solved Which of the graphs below shows the relationship
You use the standard enthalpy of the reaction and the enthalpies of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. We can evaluate the standard molar.
Standard Molar Enthalpy of Formation Calculations A Chemistry
Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. You use the standard enthalpy of the reaction and the enthalpies of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. We can evaluate the standard molar.
Definition And Explanation Of The Terms Standard State And Standard Enthalpy Of Formation, With Listing Of Values For Standard Enthalpy And.
How do you calculate standard molar enthalpy of formation? You use the standard enthalpy of the reaction and the enthalpies of. We can evaluate the standard molar gibbs energy of formation of a substance, then, from its standard molar enthalpy of formation and the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and.