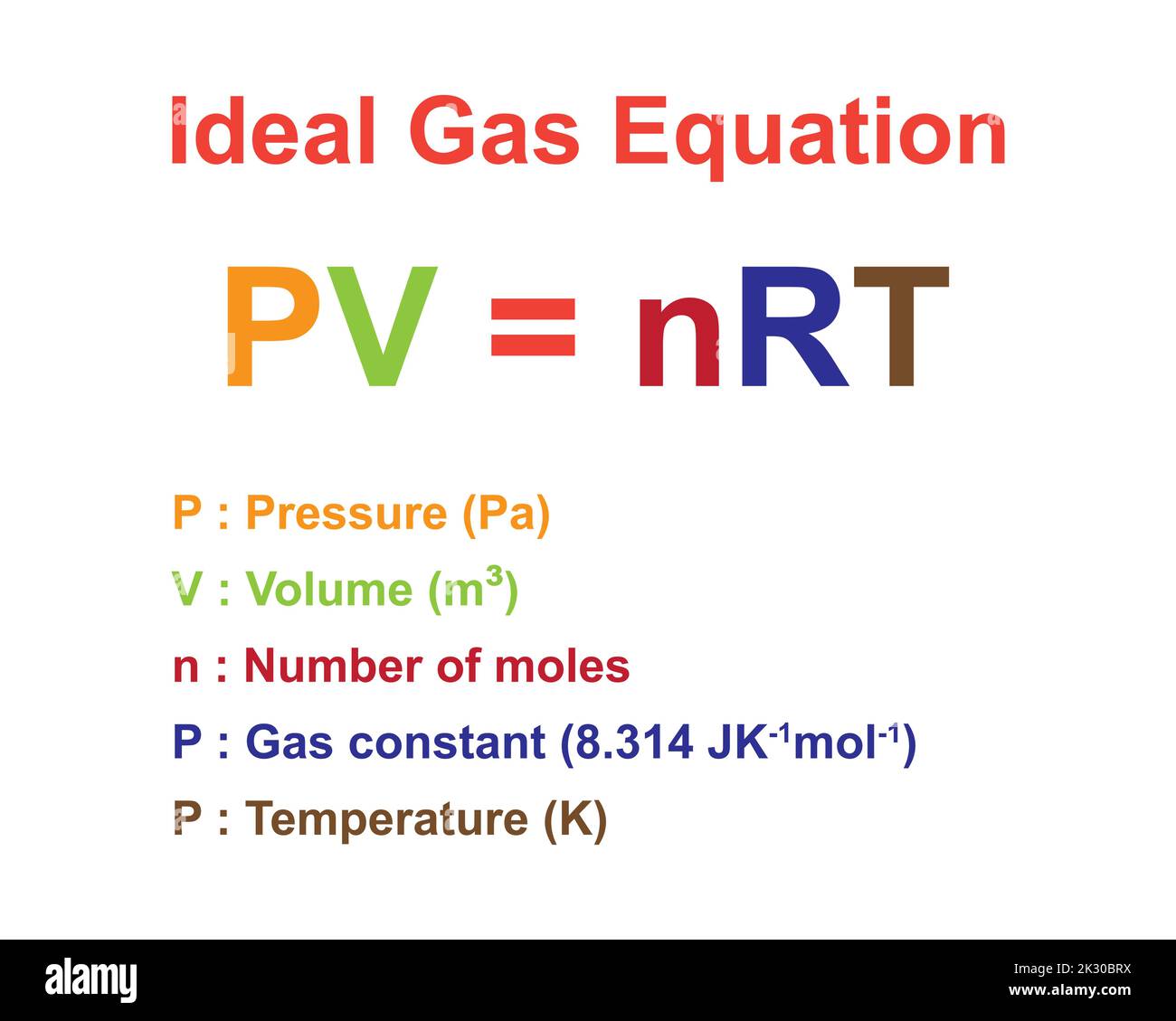
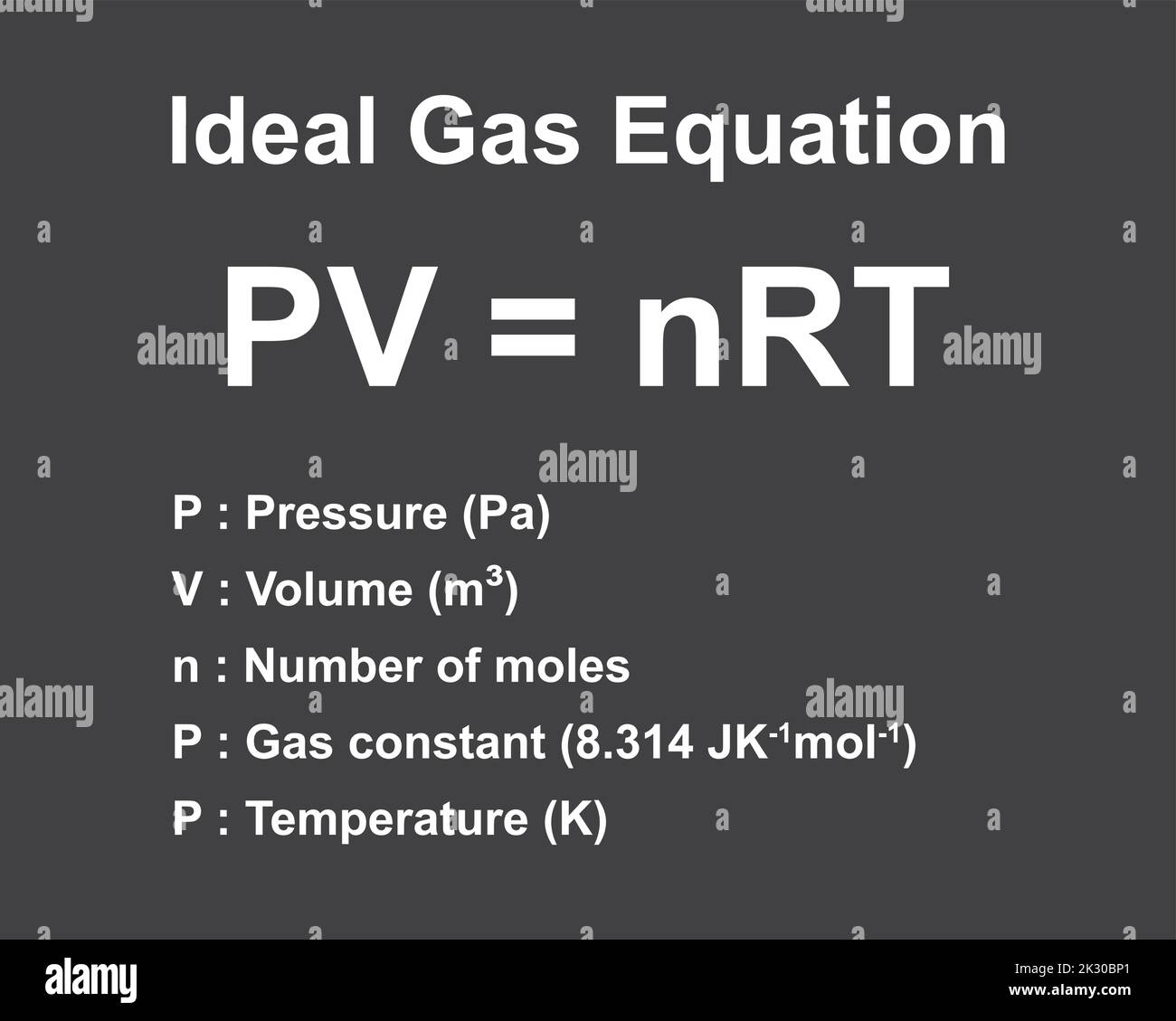
Pv Nrt Khan Academy
Pv Nrt Khan Academy - Khan academy is a 501 (c) (3) nonprofit organization. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases. For example, take pv = nrt. Understand that increasing temperature increases pressure and volume, and all you have to do to understand. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. An ideal gas is a gas in which the particles (a) do.
About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. An ideal gas is a gas in which the particles (a) do. Khan academy is a 501 (c) (3) nonprofit organization. Understand that increasing temperature increases pressure and volume, and all you have to do to understand. For example, take pv = nrt. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases.
Understand that increasing temperature increases pressure and volume, and all you have to do to understand. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. Khan academy is a 501 (c) (3) nonprofit organization. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases. An ideal gas is a gas in which the particles (a) do. For example, take pv = nrt.
PV NRT chemistry Chemistry TShirt TeePublic
For example, take pv = nrt. Khan academy is a 501 (c) (3) nonprofit organization. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases.
paciente Prevalecer Claire ley de los gases ideales pv nrt pivote
Understand that increasing temperature increases pressure and volume, and all you have to do to understand. For example, take pv = nrt. Khan academy is a 501 (c) (3) nonprofit organization. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. The ideal gas law (pv = nrt) relates the macroscopic properties.
As always… LonCapa assignments Lecture videos Textbook Read ppt download
Understand that increasing temperature increases pressure and volume, and all you have to do to understand. An ideal gas is a gas in which the particles (a) do. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. Khan academy is a 501 (c) (3) nonprofit organization. The ideal gas law (pv.
Solved] Using The Ideal Gas Law, PV NRT, Where R L Atm/mol , 56 OFF
About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. Understand that increasing temperature increases pressure and volume, and all you have to do to understand. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases..
Understanding the Ideal Gas Theory How It Affects Your Tire Pressure
For example, take pv = nrt. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. Understand that increasing temperature increases pressure and volume, and all.
PV = nRT Ideal Gas Law Brings Together Gas Properties. The Most
The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. For example, take pv = nrt. Khan academy is a 501 (c) (3) nonprofit organization. Understand that increasing temperature increases pressure and volume, and all you have to.
Ideal gas equation PV = nRT Chemistry Khan Academy Chemistry
Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. Understand that increasing temperature increases pressure and volume, and all you have to do to understand. An ideal gas is a gas in which the particles (a) do. For example, take pv = nrt. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases.
How to prove that pv=nrt?
For example, take pv = nrt. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. Understand that increasing temperature increases pressure and volume, and all you have to do to understand. An ideal gas is a gas.
Ideal Gas Law — Overview & Calculations Expii
An ideal gas is a gas in which the particles (a) do. Understand that increasing temperature increases pressure and volume, and all you have to do to understand. For example, take pv = nrt. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases. About press copyright contact us creators advertise developers terms privacy policy &.
Nrt Black and White Stock Photos & Images Alamy
Khan academy is a 501 (c) (3) nonprofit organization. The ideal gas law (pv = nrt) relates the macroscopic properties of ideal gases. An ideal gas is a gas in which the particles (a) do. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard. About press copyright contact us creators advertise developers terms privacy policy & safety.
Khan Academy Is A 501 (C) (3) Nonprofit Organization.
For example, take pv = nrt. An ideal gas is a gas in which the particles (a) do. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works press copyright. Khan academy offers practice exercises, instructional videos, and a personalized learning dashboard.
The Ideal Gas Law (Pv = Nrt) Relates The Macroscopic Properties Of Ideal Gases.
Understand that increasing temperature increases pressure and volume, and all you have to do to understand.



![Solved] Using The Ideal Gas Law, PV NRT, Where R L Atm/mol , 56 OFF](https://i.ytimg.com/vi/aF2ei1XCxTg/maxresdefault.jpg)