Is Forming Bonds Exothermic
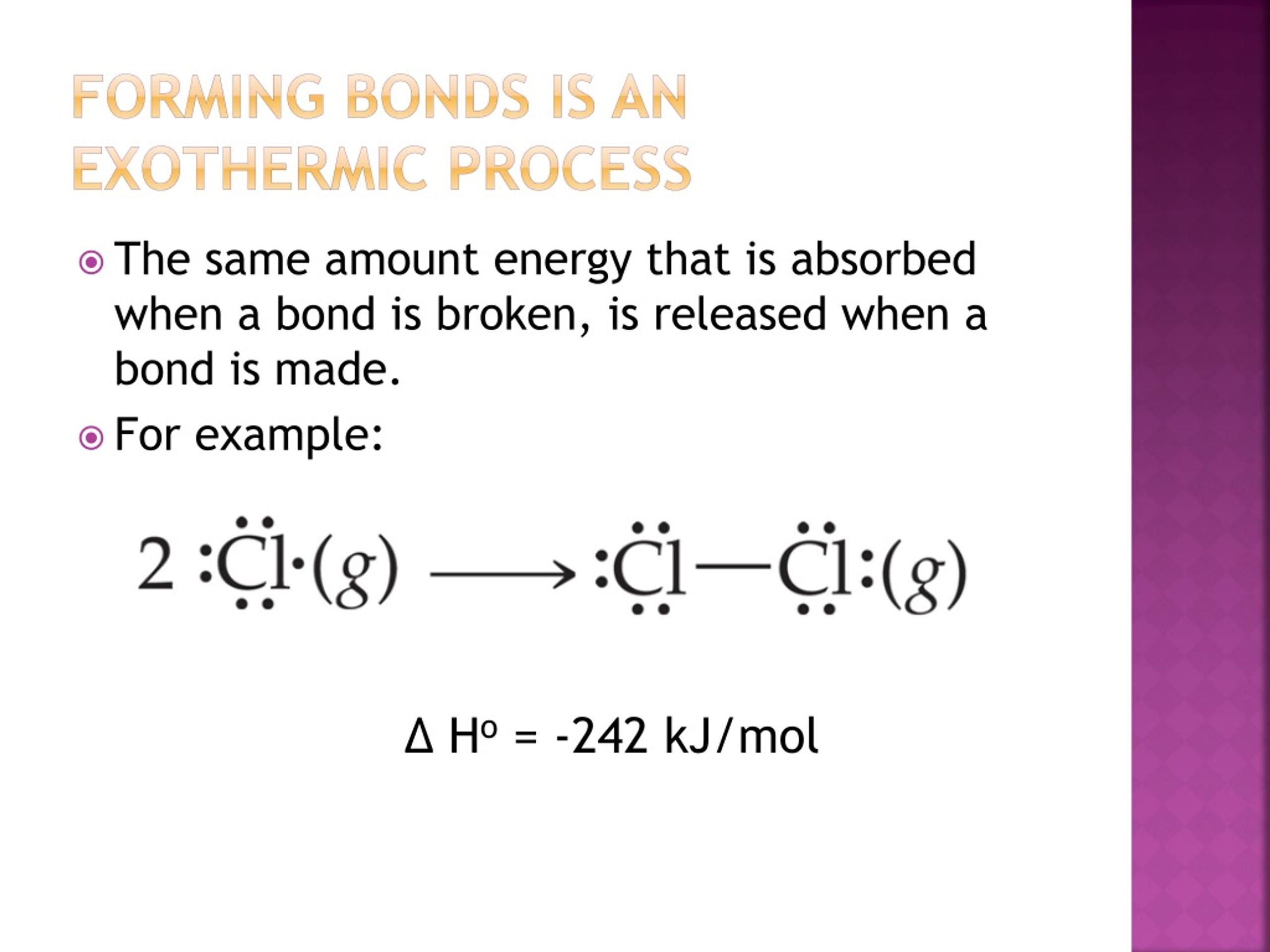
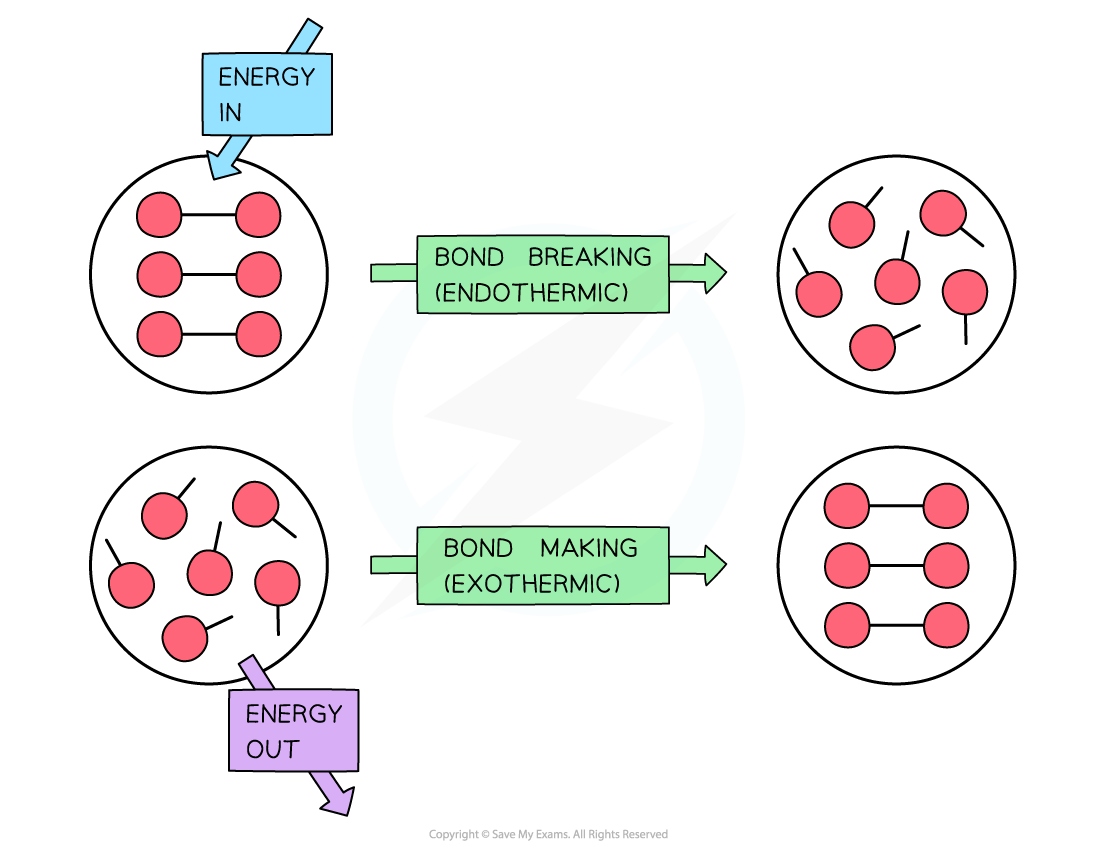
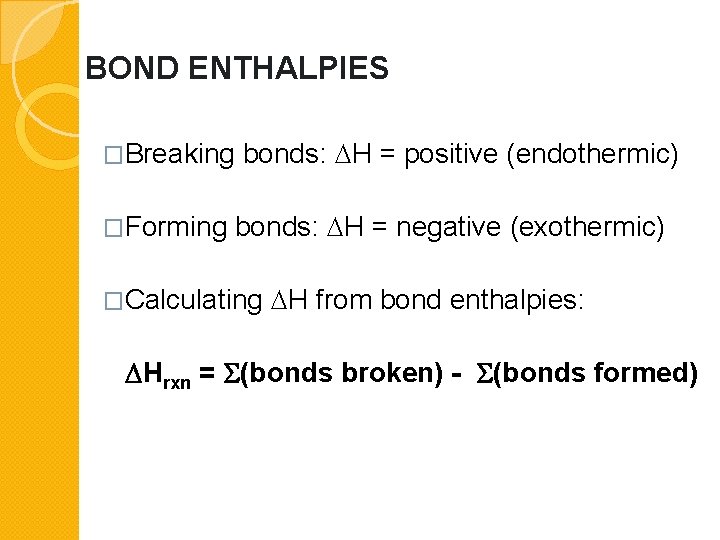
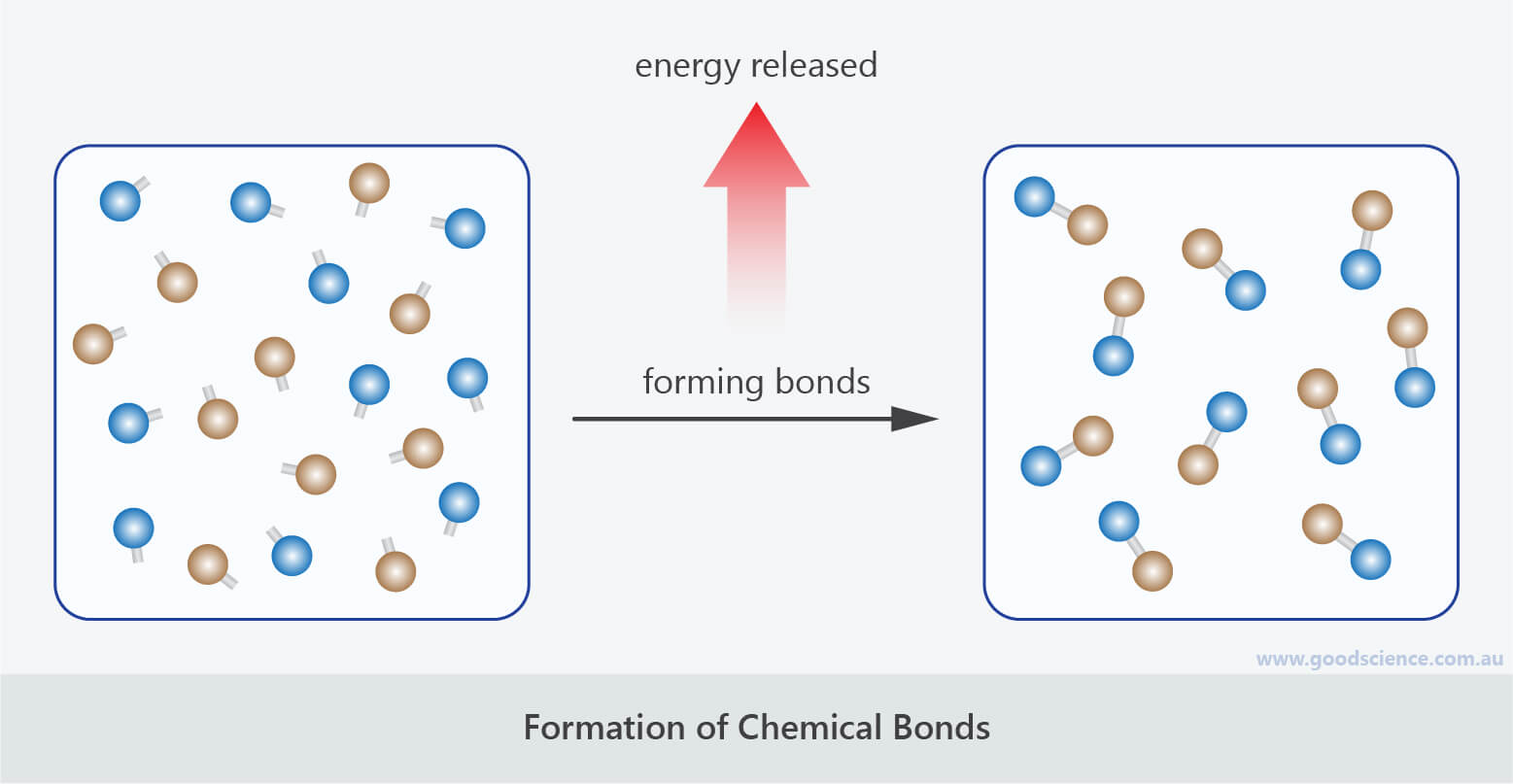
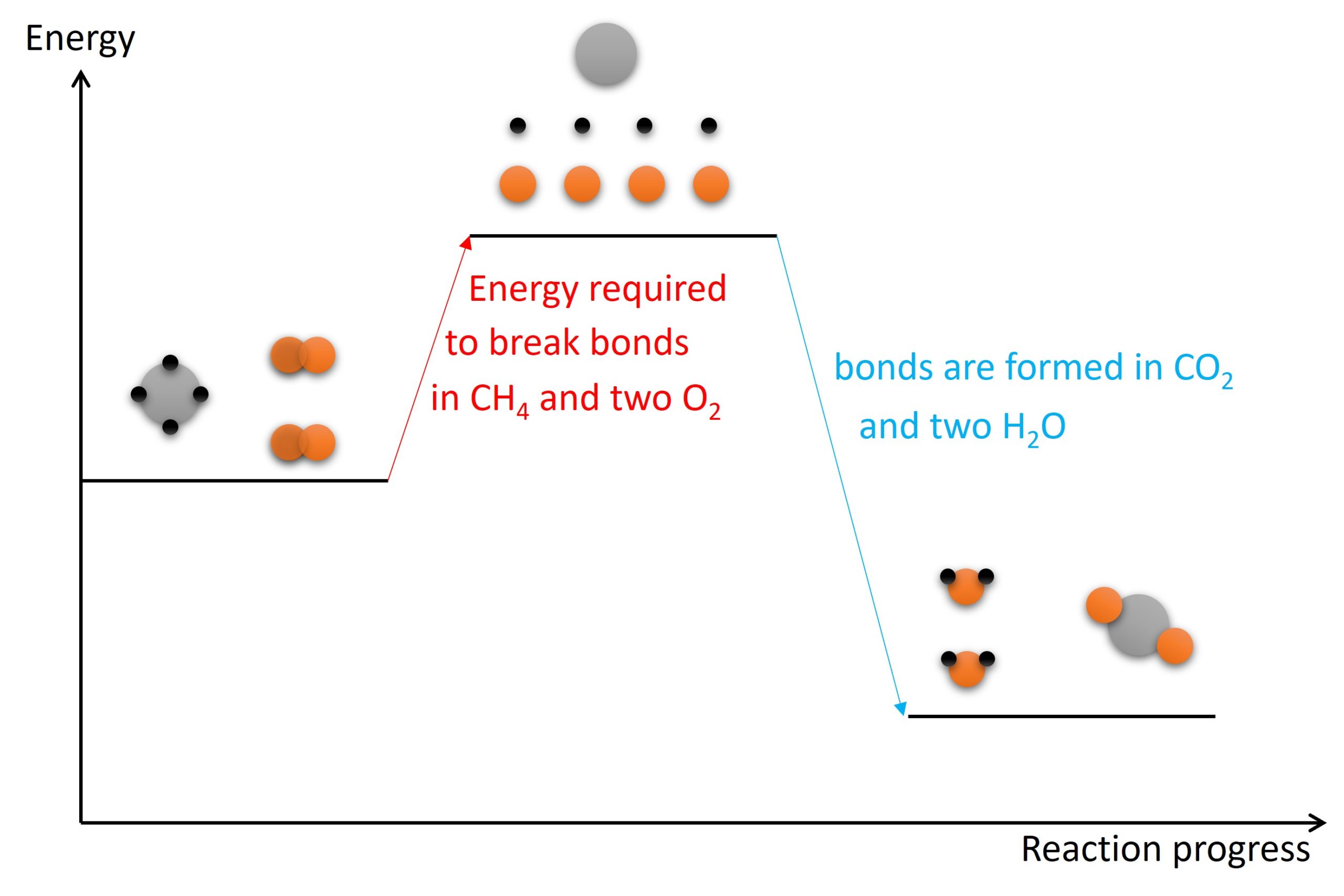
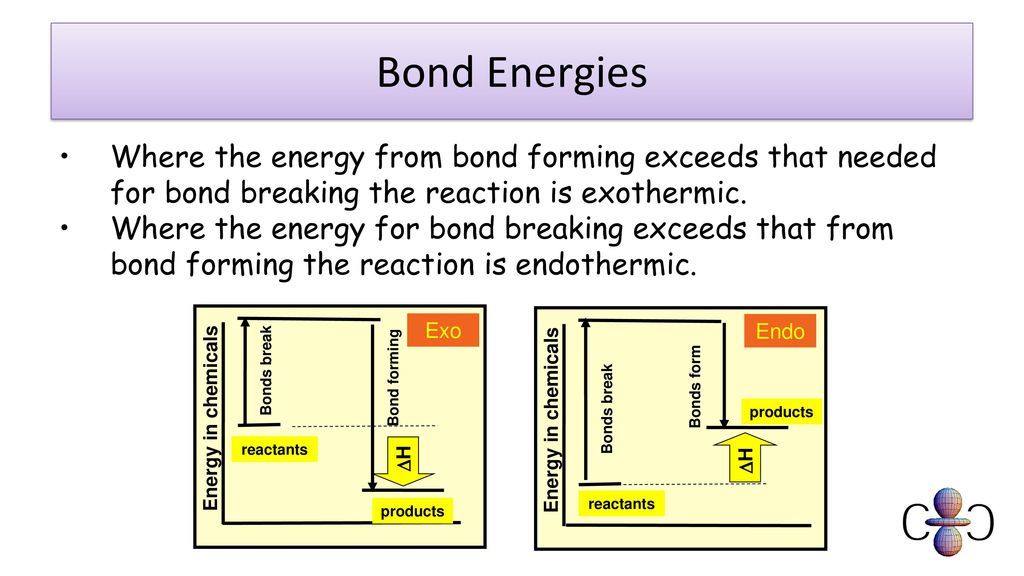
Is Forming Bonds Exothermic - Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and.
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not:
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:.
SOLVED Which of these is the TRUE statement? A) Combustion reactions
In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and.
PPT Energetics PowerPoint Presentation, free download ID9217507
In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:.
Edexcel A Level Chemistry复习笔记1.8.5 Bond Enthalpy翰林国际教育
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:.
Energetics IB Topics 5 15 PART 2 Calculating
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:.
Exothermic and Endothermic Reactions and the Math Associated with Them
In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and.
Investigating Chemical Reactions Good Science
Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and.
5.1 Exothermic and endothermic reactions IGCSE and A Level Chemistry
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not:
Bond energy
Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not:
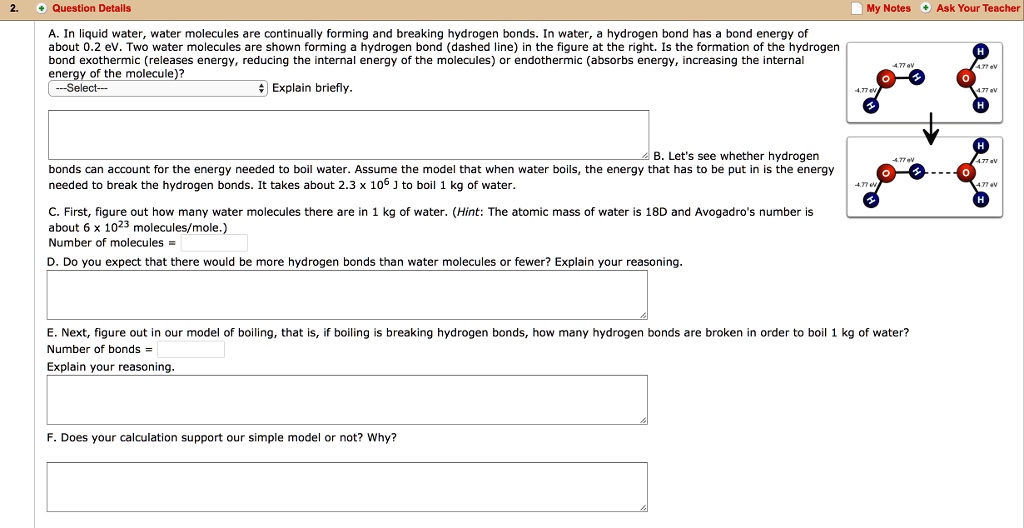
SOLVED " In liquid water, water molecules are continually forming and
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and. Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not:
Energy changes in chemistry bond enthalpies ppt download
Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. In summary, there are two factors which determine whether a gaseous reaction will be exothermic or not: Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and.
In Summary, There Are Two Factors Which Determine Whether A Gaseous Reaction Will Be Exothermic Or Not:
Now that we have a basic understanding of exothermic reactions and bond enthalpy, let’s get back to the question at hand:. Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and.