In What Form Can An Ionic Compound Conduct Electricity
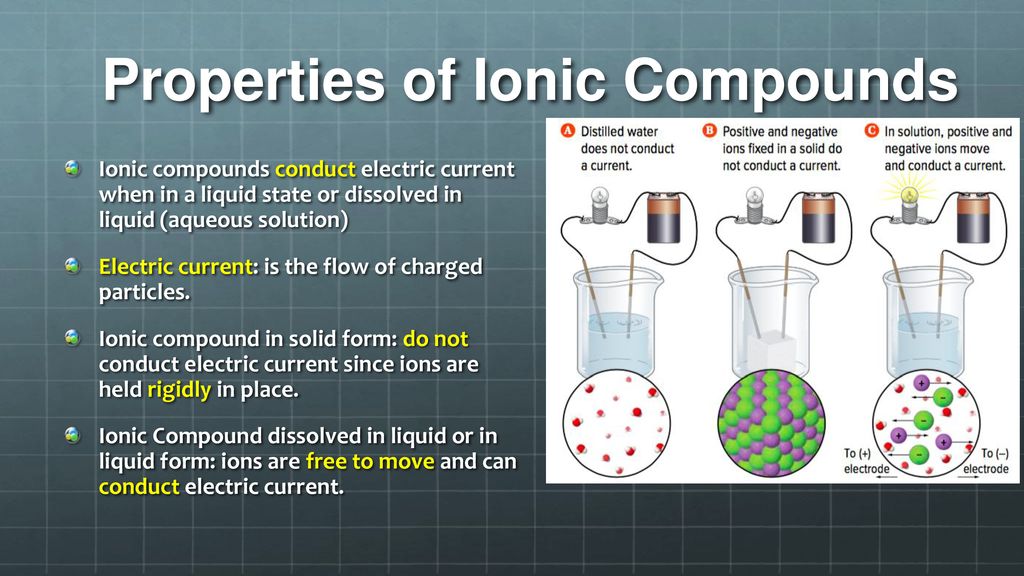
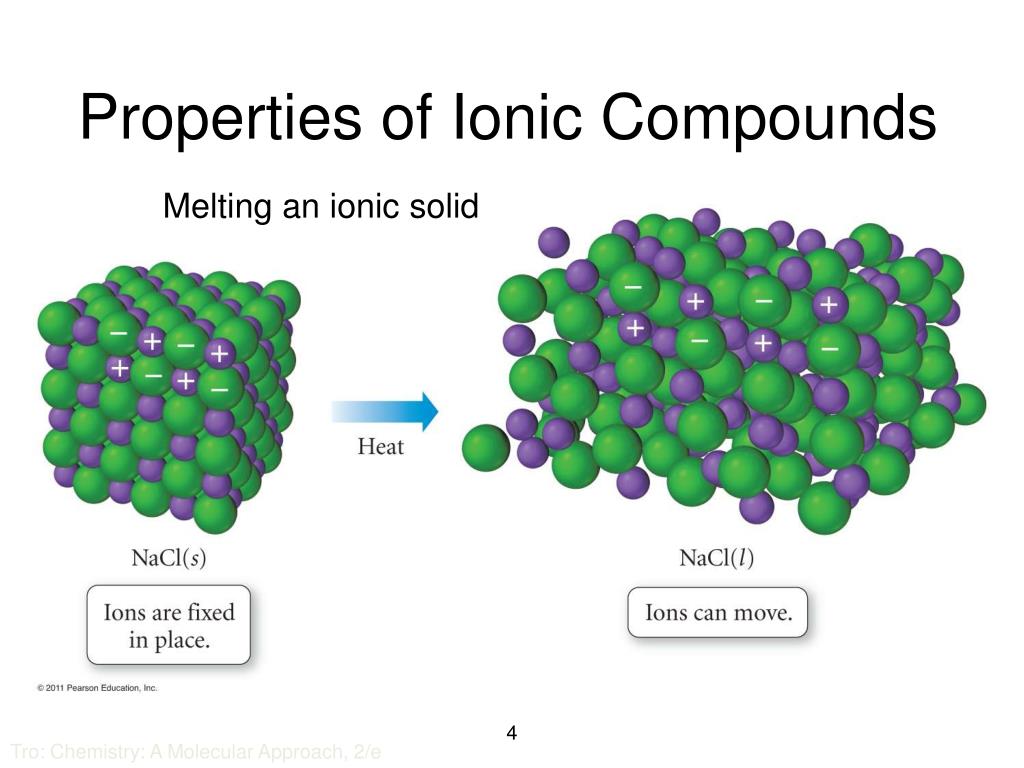
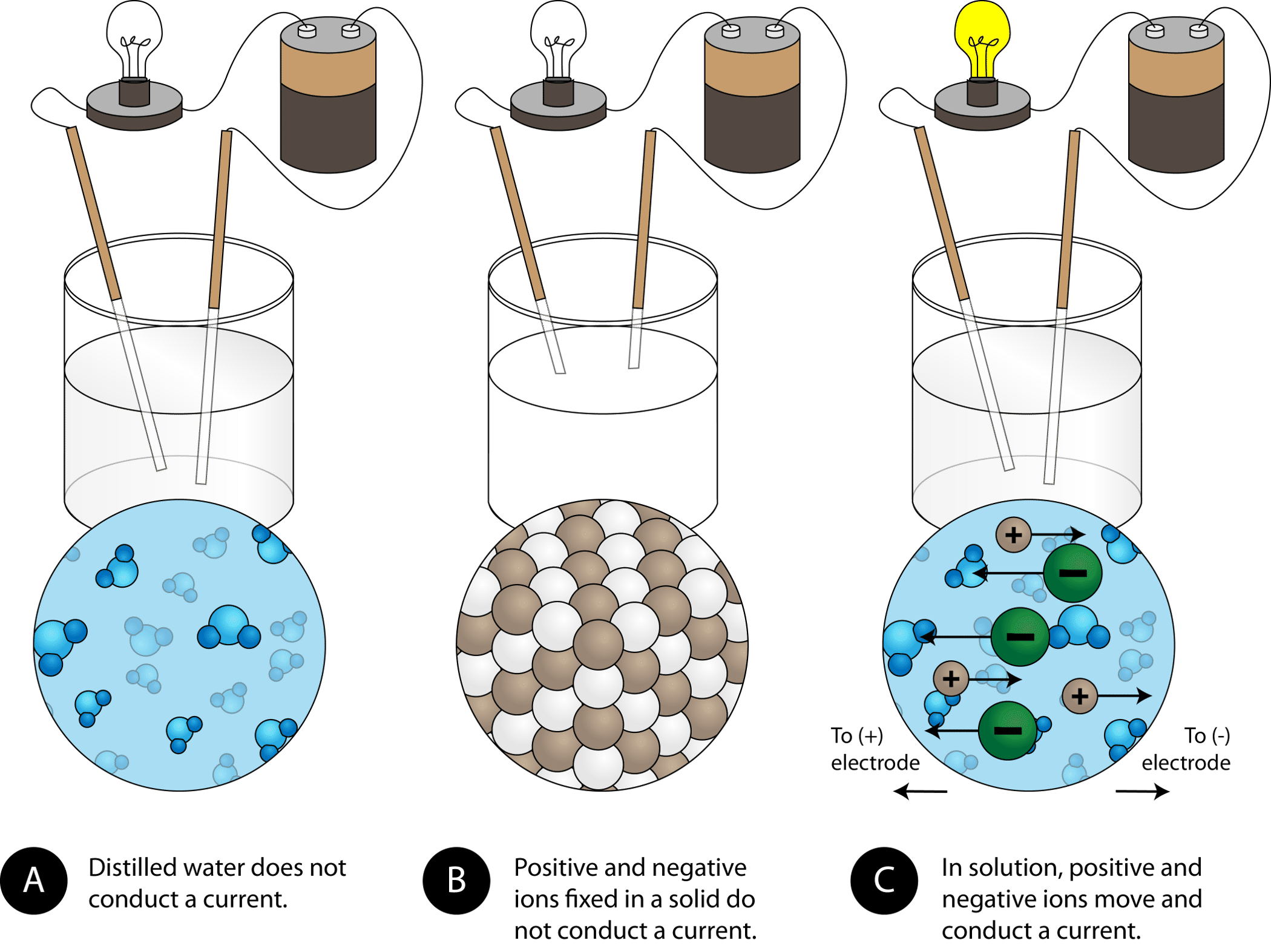
In What Form Can An Ionic Compound Conduct Electricity - Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Learn about and revise ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. They are insulators when solid. Ionic compounds conduct electricity when melted or in solution. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds.
Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Ionic compounds conduct electricity when melted or in solution. They are insulators when solid. Learn about and revise ionic compounds. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds.
Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ionic compounds conduct electricity when melted or in solution. Learn about and revise ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. They are insulators when solid.
What Is An Ionic Compound? Formula and Defination
Ionic compounds conduct electricity when melted or in solution. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Learn about and revise ionic compounds. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ions of opposite charges are tightly packed together to create.
Ionic Bonding. ppt download
In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Learn about and revise ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic.
How Ionic Compounds Conduct Electricity GCSE Chemistry kayscience
In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Ionic compounds conduct electricity when.
Properties of ionic compounds Chemistry Quizizz
Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Learn about and revise ionic compounds. Ionic compounds conduct electricity when melted or in solution. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. They are insulators when solid.
What are Ionic Compounds and how they are formed?
Learn about and revise ionic compounds. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. They are insulators when solid. Ionic compounds conduct electricity when melted or in solution. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid.
Would The Resulting Solution Conduct Electricity SAEQVU
Ionic compounds conduct electricity when melted or in solution. They are insulators when solid. Learn about and revise ionic compounds. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid.
Ionic Properties
In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. They are insulators when solid. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds..
Ionic Bond — Formation & Compounds Expii
Learn about and revise ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. They are insulators when solid. Ionic compounds conduct electricity when melted or in solution.
Properties of Ionic Compounds GCSE Chemistry Revision
They are insulators when solid. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Learn about and revise ionic compounds. Ionic compounds conduct electricity when melted or in solution.
How Do Ions Conduct Electricity
They are insulators when solid. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Learn about and revise ionic compounds. Ionic compounds conduct electricity when melted or in solution.
They Are Insulators When Solid.
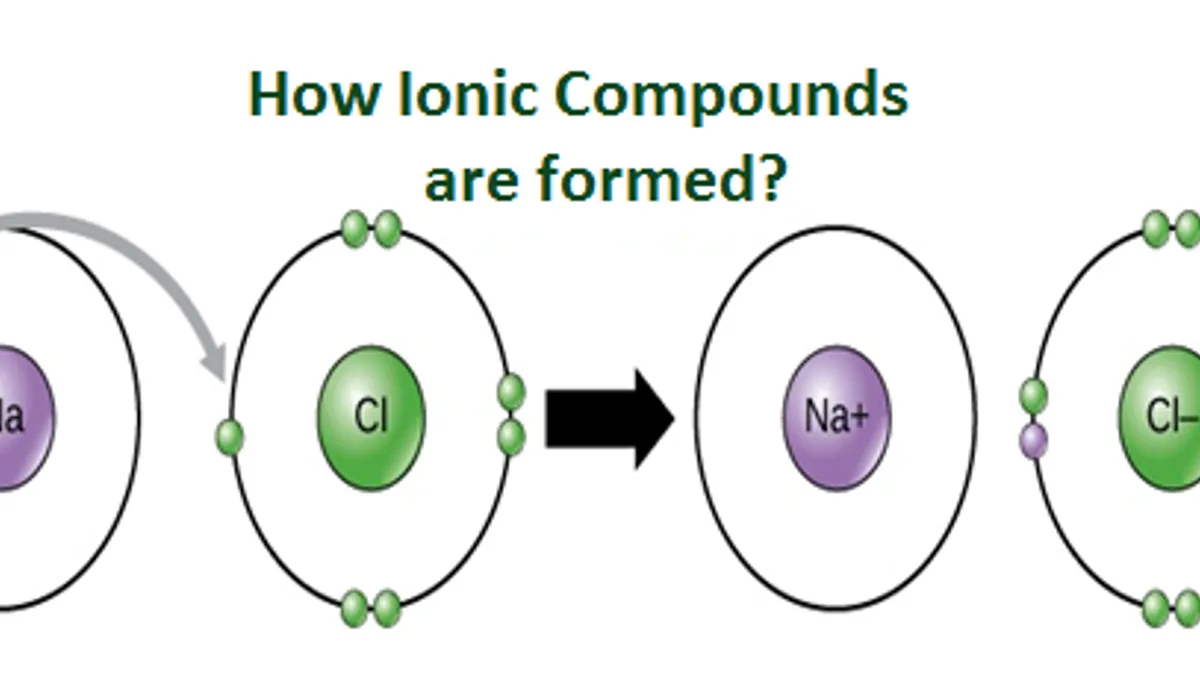
Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds conduct electricity when melted or in solution. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid.
Learn About And Revise Ionic Compounds.
In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the.