How Many Hydrogen Bonds Can A Water Molecule Form
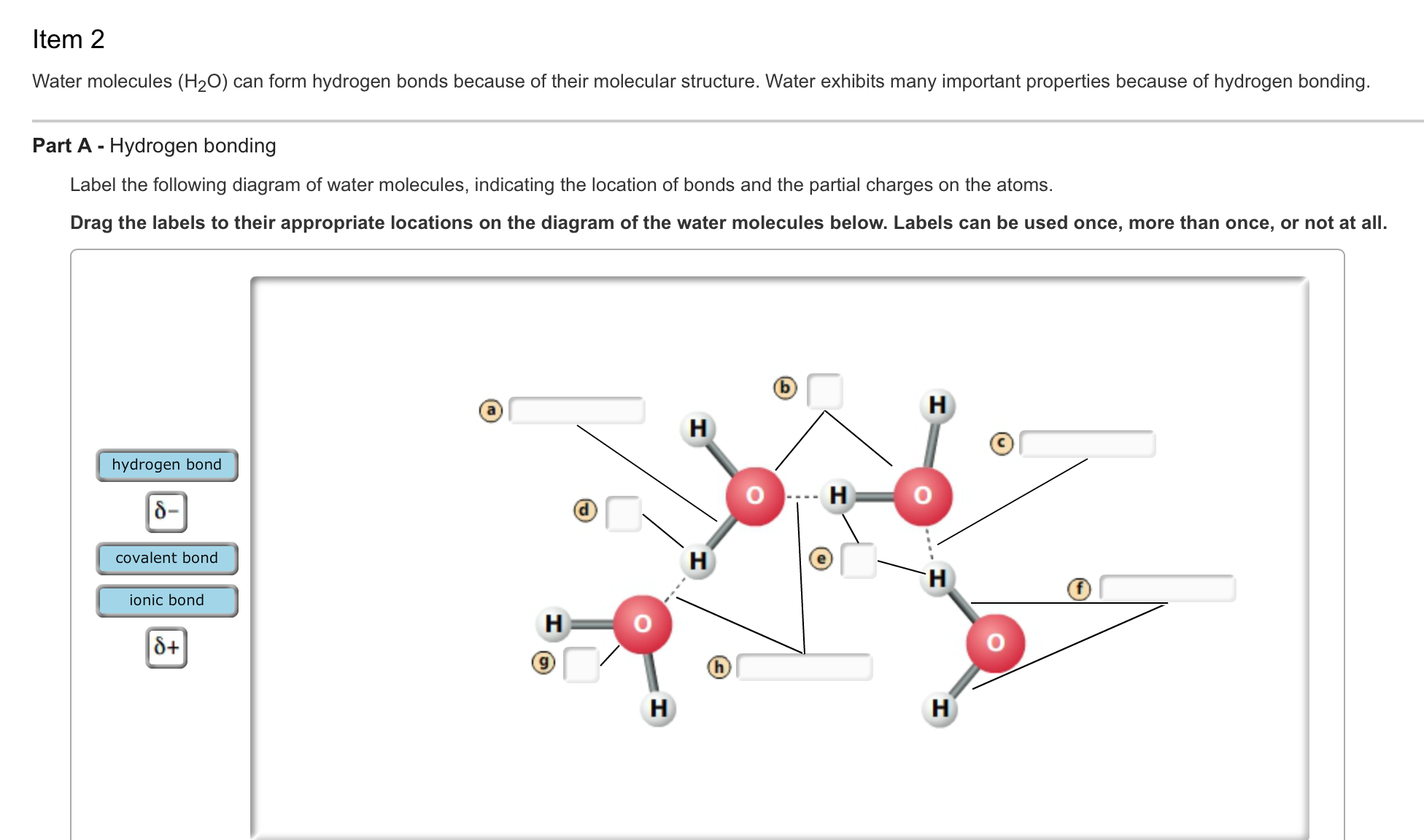
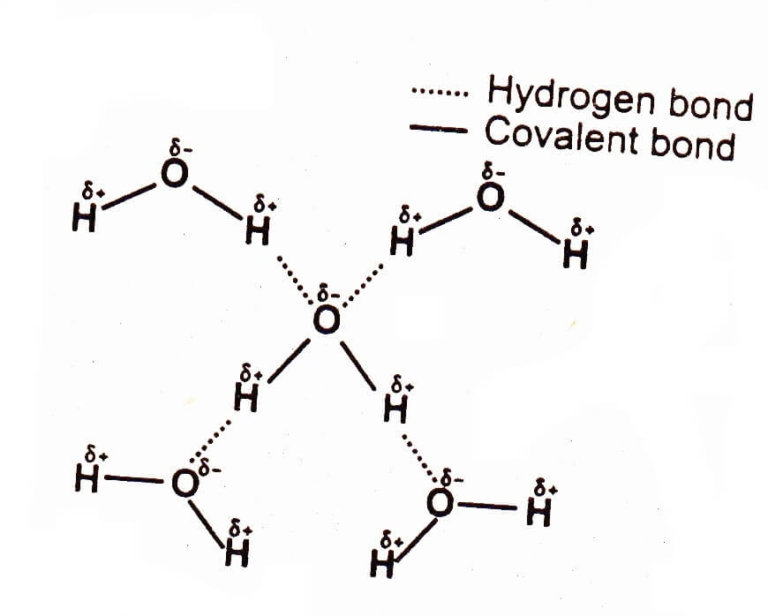
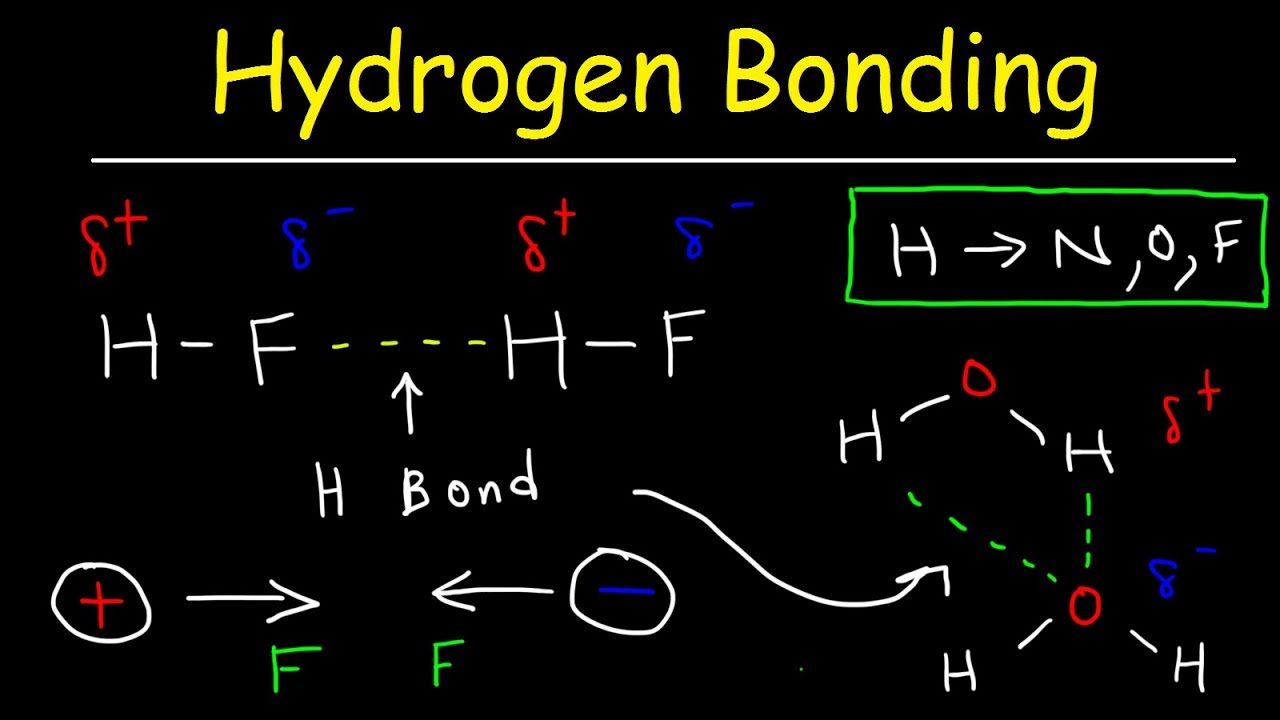
How Many Hydrogen Bonds Can A Water Molecule Form - The oxygen atom has a slightly negative. A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. How many hydrogen bonds can a single water molecule form? A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Both an oxygen atom and 2 hydrogen atoms in one. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Positive hydrogen of one molecule attracted to negative oxygen of nearby.
Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Positive hydrogen of one molecule attracted to negative oxygen of nearby. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Both an oxygen atom and 2 hydrogen atoms in one. The oxygen atom has a slightly negative. A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. How many hydrogen bonds can a single water molecule form? A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom.
The oxygen atom has a slightly negative. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. How many hydrogen bonds can a single water molecule form? Positive hydrogen of one molecule attracted to negative oxygen of nearby. Both an oxygen atom and 2 hydrogen atoms in one. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules.
how many hydrogen bonds can a single water molecule form
A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. The oxygen atom has a slightly negative. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Positive hydrogen of one molecule attracted to negative oxygen of nearby. How many hydrogen bonds can a single water molecule form?
learning through art water molecules and hydrogen bonding
A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. The oxygen atom has a slightly negative. Both an oxygen atom and 2 hydrogen atoms in one. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Positive hydrogen of one molecule.
Hydrogen Bonding Chemistry Skills
Both an oxygen atom and 2 hydrogen atoms in one. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. How many hydrogen bonds can a single water molecule form? Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. A single.
Download How Many Hydrogen Bonds Can A Water Molecule Form Hydrogen
A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. How many hydrogen bonds can a single water molecule form? The oxygen atom has a slightly negative. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. A single water molecule can form.
Senyawa dan Ikatan Kimia Warung Sains Teknologi
Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Positive hydrogen of one molecule attracted to negative oxygen of nearby. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. A water molecule (h2o) consists of two hydrogen atoms bonded to.
Hydrogen bonding Hopinno
Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Positive hydrogen of one molecule attracted to negative oxygen of nearby. Both an oxygen atom and 2 hydrogen atoms in one. A single water molecule can form a maximum.
Diagram Of Water Molecules Hydrogen Bonding
How many hydrogen bonds can a single water molecule form? A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Positive hydrogen of one molecule attracted to negative oxygen of nearby. Both an oxygen atom and 2 hydrogen atoms.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
How many hydrogen bonds can a single water molecule form? Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Both an oxygen atom and 2 hydrogen atoms in one. Positive hydrogen.
The importance of the water and its structure Science online
The oxygen atom has a slightly negative. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. A single water molecule can form a maximum of four hydrogen bonds with neighboring water.
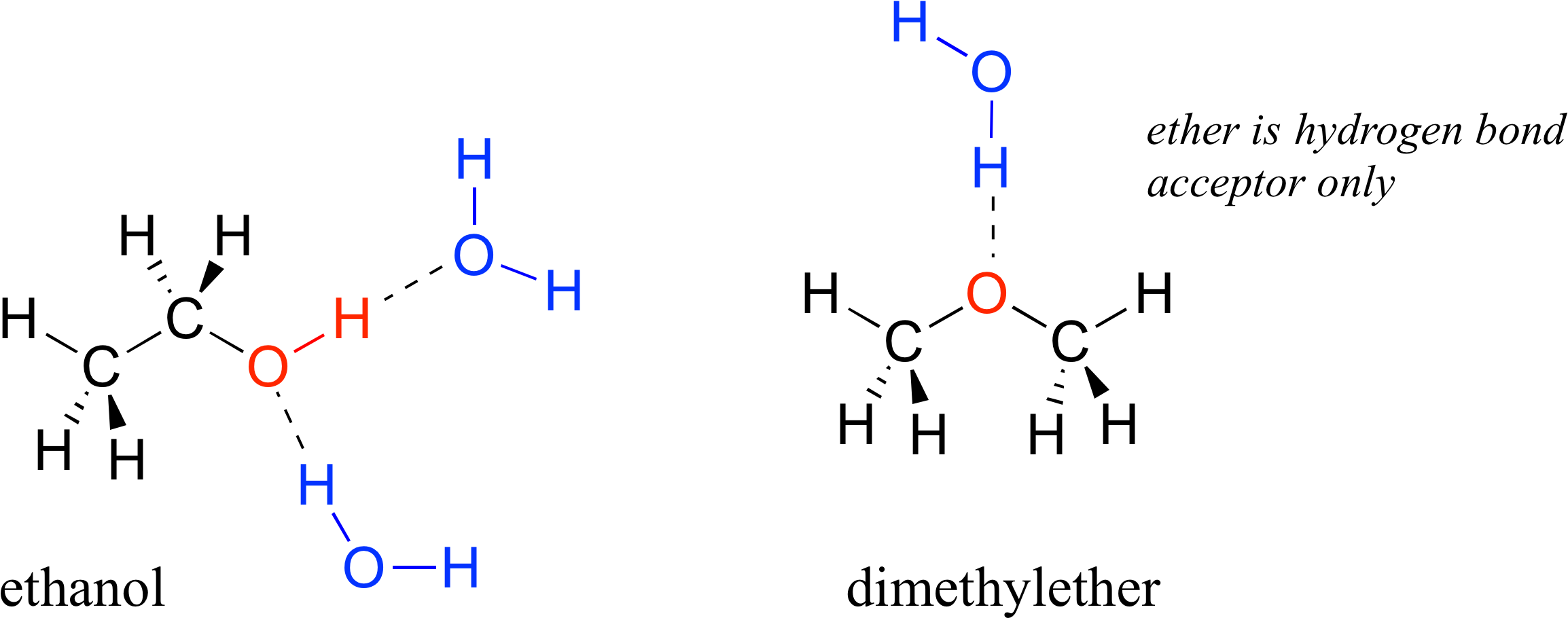
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Both an oxygen atom and 2 hydrogen.
Both An Oxygen Atom And 2 Hydrogen Atoms In One.
A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. The oxygen atom has a slightly negative. A single water molecule can form a maximum of four hydrogen bonds with neighboring water molecules. How many hydrogen bonds can a single water molecule form?
Up To 4 Hydrogen Bonds Can Form Between A Single Water Molecule And Other Water Molecules.
Positive hydrogen of one molecule attracted to negative oxygen of nearby. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule.