How Many Bonds Can Carbon Form
How Many Bonds Can Carbon Form - Carbon is able to form long, stable backbones because of its small size and bond flexibility. The valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since carbon has a valency of 4 it can. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. The number of bonds an atom can form depends on that atom's valence electrons. Carbon is an essential element for life on earth, as it can easily form bonds with other atoms to create the proteins, carbohydrates, and lipids needed by living organisms. Carbon is an incredibly versatile molecule that can form many kinds of bonds with different atoms. An atom can go any distance to complete its octet! Carbon can form either 2 or 4 bonds.
The number of bonds an atom can form depends on that atom's valence electrons. Carbon can form either 2 or 4 bonds. Carbon is able to form long, stable backbones because of its small size and bond flexibility. Carbon is an essential element for life on earth, as it can easily form bonds with other atoms to create the proteins, carbohydrates, and lipids needed by living organisms. The valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since carbon has a valency of 4 it can. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. Carbon is an incredibly versatile molecule that can form many kinds of bonds with different atoms. An atom can go any distance to complete its octet!
Carbon can form either 2 or 4 bonds. An atom can go any distance to complete its octet! Carbon is an essential element for life on earth, as it can easily form bonds with other atoms to create the proteins, carbohydrates, and lipids needed by living organisms. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. The number of bonds an atom can form depends on that atom's valence electrons. Carbon is able to form long, stable backbones because of its small size and bond flexibility. The valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since carbon has a valency of 4 it can. Carbon is an incredibly versatile molecule that can form many kinds of bonds with different atoms.
How many bonds can Carbon form? Why is this important? GufoSaggio
The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. Carbon is an incredibly versatile molecule that can form many kinds of bonds with different atoms. Carbon can form either 2 or 4 bonds. An atom can go any distance to complete its octet! The number.
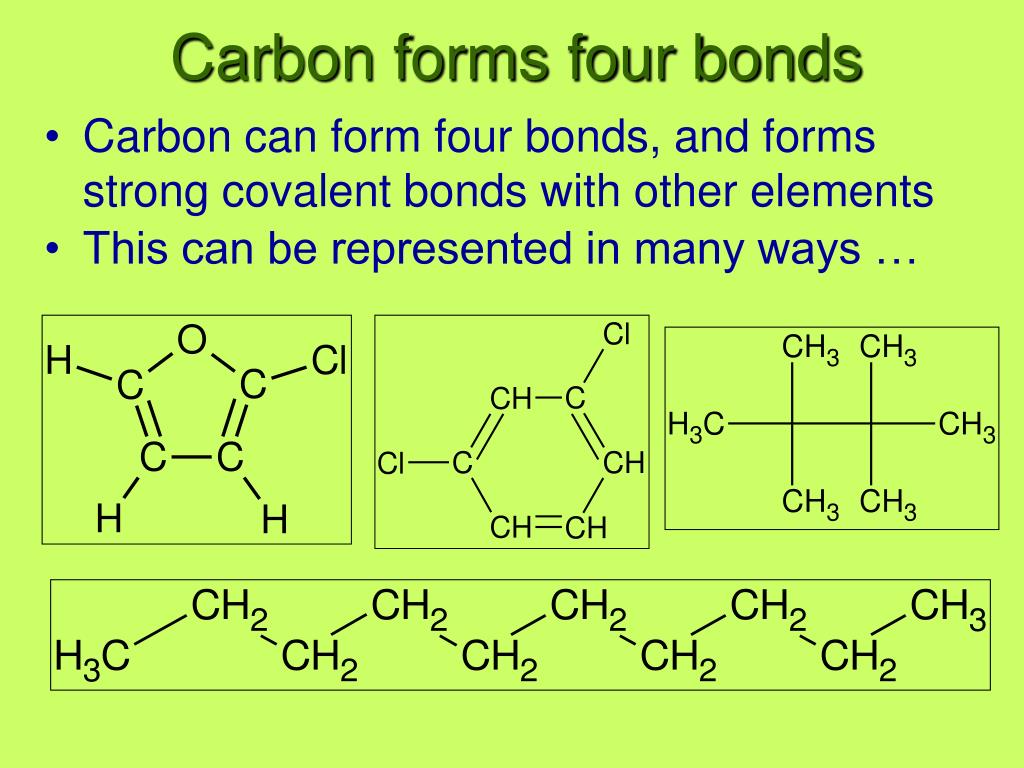
Chapter 4 Carbon Do Now How many bonds can carbon form? ppt download
Carbon is able to form long, stable backbones because of its small size and bond flexibility. An atom can go any distance to complete its octet! The number of bonds an atom can form depends on that atom's valence electrons. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which.
How many bonds can carbon form
Carbon is able to form long, stable backbones because of its small size and bond flexibility. An atom can go any distance to complete its octet! Carbon is an essential element for life on earth, as it can easily form bonds with other atoms to create the proteins, carbohydrates, and lipids needed by living organisms. The ground state electron configuration.
Chapter 4 Carbon Do Now How many bonds can carbon form? ppt download
Carbon is able to form long, stable backbones because of its small size and bond flexibility. Carbon can form either 2 or 4 bonds. Carbon is an incredibly versatile molecule that can form many kinds of bonds with different atoms. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which.
How many bonds can Carbon form?
Carbon is able to form long, stable backbones because of its small size and bond flexibility. The number of bonds an atom can form depends on that atom's valence electrons. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. Carbon is an essential element for.
How many bonds can carbon form?
The number of bonds an atom can form depends on that atom's valence electrons. Carbon is able to form long, stable backbones because of its small size and bond flexibility. An atom can go any distance to complete its octet! The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which.
Double Covalent Bond Covalent Bonding Quiz ProProfs Quiz, The
The number of bonds an atom can form depends on that atom's valence electrons. Carbon is able to form long, stable backbones because of its small size and bond flexibility. Carbon is an incredibly versatile molecule that can form many kinds of bonds with different atoms. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon.
__TOP__ How Many Covalent Bonds Can Chlorine Form
The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. The number of bonds an atom can form depends on that atom's valence electrons. An atom can go any distance to complete its octet! Carbon is an incredibly versatile molecule that can form many kinds of.
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
The valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since carbon has a valency of 4 it can. Carbon can form either 2 or 4 bonds. Carbon is an essential element for life on earth, as it can easily form bonds with other.
PPT Organic Chemistry PowerPoint Presentation, free download ID5887001
Carbon is able to form long, stable backbones because of its small size and bond flexibility. Carbon can form either 2 or 4 bonds. The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. The number of bonds an atom can form depends on that atom's.
The Valency Of An Element Tells Us How Much Atoms Do The Atom Of That Particular Element Needs To Achieve A Stable Electronic Configuration So, Here Since Carbon Has A Valency Of 4 It Can.
The number of bonds an atom can form depends on that atom's valence electrons. An atom can go any distance to complete its octet! The ground state electron configuration is 1s^2 2s^2 2p^2 in this ground state carbon has 2 unpaired p electrons which can form 2 bonds. Carbon can form either 2 or 4 bonds.
Carbon Is An Incredibly Versatile Molecule That Can Form Many Kinds Of Bonds With Different Atoms.
Carbon is able to form long, stable backbones because of its small size and bond flexibility. Carbon is an essential element for life on earth, as it can easily form bonds with other atoms to create the proteins, carbohydrates, and lipids needed by living organisms.




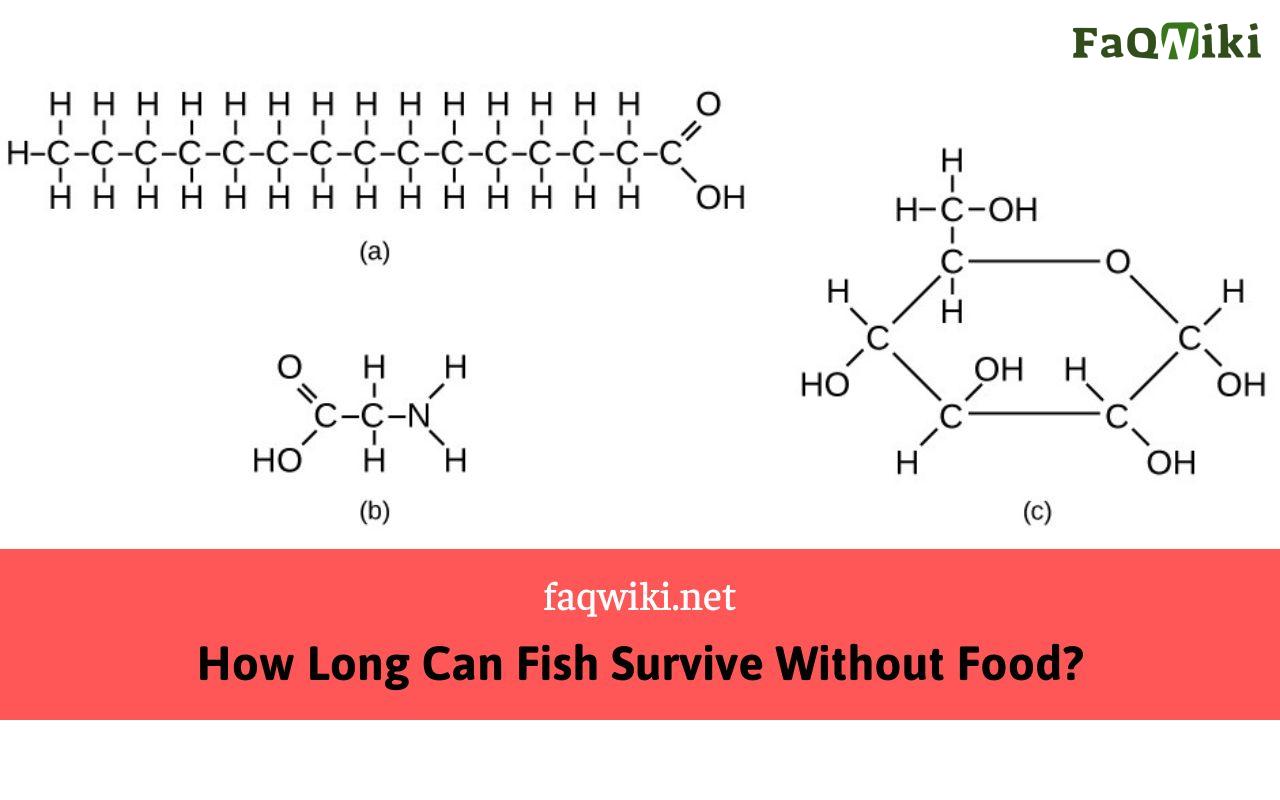



![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://d1avenlh0i1xmr.cloudfront.net/large/6f2704db-da18-4198-8dd5-4dc81ae3ac22/carbon-sharing-electrons-with-hydrogen---teachoo.jpg)