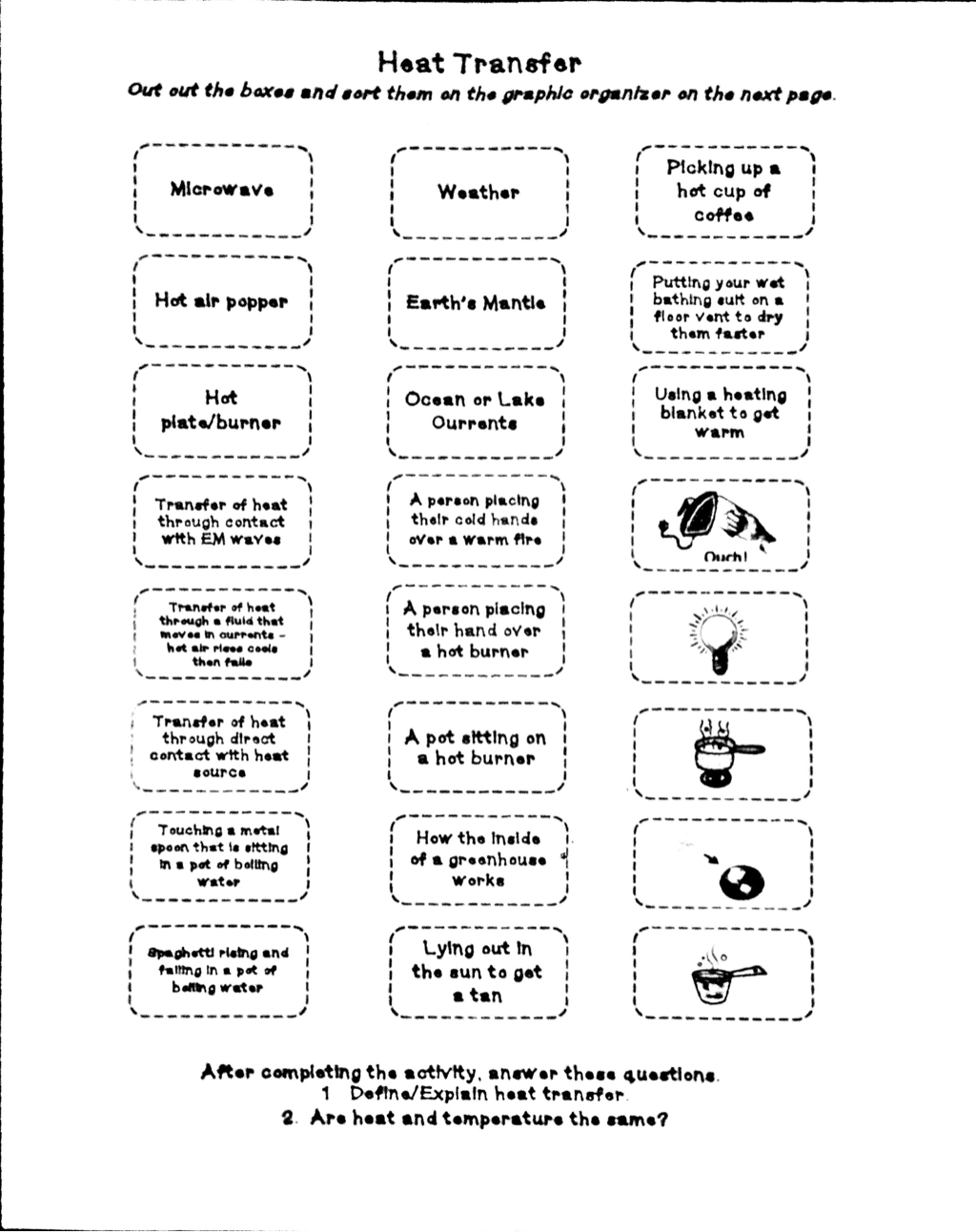
Heat Transfer Specific Heat Problems Worksheet
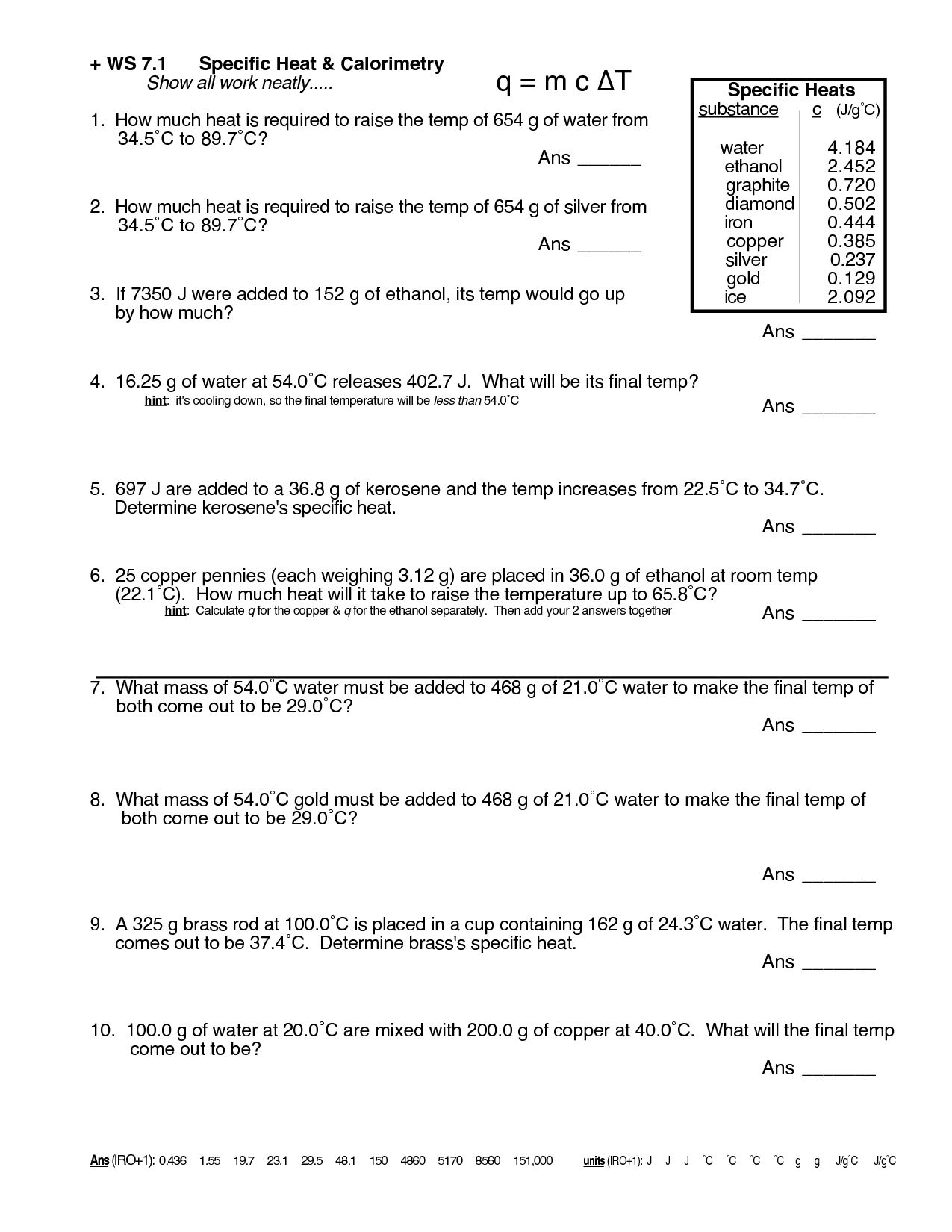
Heat Transfer Specific Heat Problems Worksheet - Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units.
Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Heat Transfer Worksheet Answers Englishworksheet.my.id
Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems.
Specific Heat Practice Problems Answers Page 1 of 3 Specific Heat
Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems.
Free specific heat worksheet with answers, Download Free specific heat
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Free specific heat and heat capacity worksheet, Download Free specific
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Specific Heat Worksheet Answers Master of Documents
Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Heat Transfer Worksheet Answers E Street Light
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
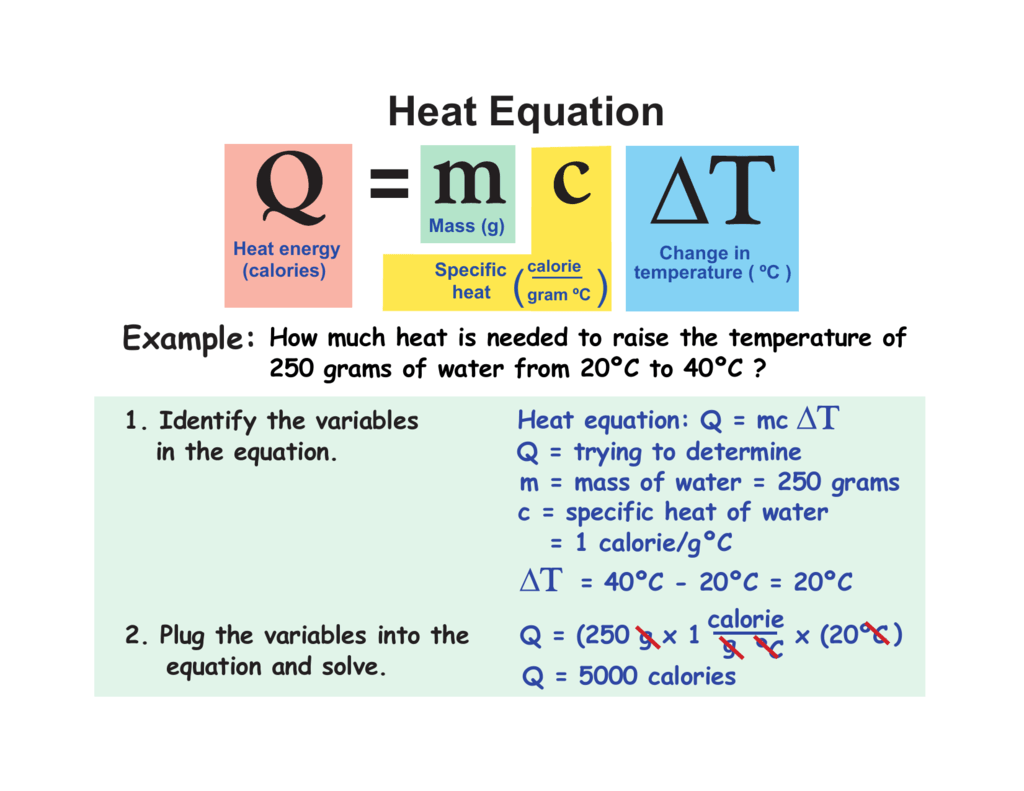
Heat Equation
Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Show all work and units.
Heat Transfer Specific Heat Problems Worksheet —
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units.
Heat Transfer/ Specific Heat Problems Worksheet
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Show all work and units. Use q = (m)(δt)(cp) to solve the following problems.
Use Q = (M)(Δt)(Cp) To Solve The Following Problems.
Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.