Fda Form 483 Definition
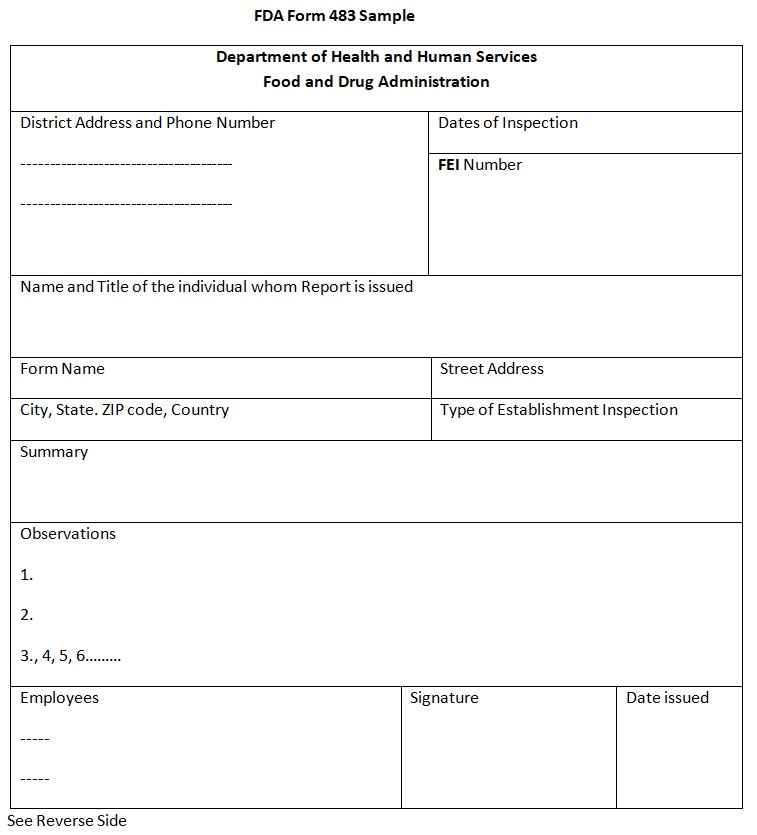
Fda Form 483 Definition - A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,.
An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,.
What is FDA Form 483? YouTube
An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
FDA Form 483 Observations and FDA Warning Letters What’s the Difference?
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. During an inspection, ora investigators may observe conditions they deem to be objectionable.
Study on FDA Form 483 observations Hownature Nutraceutical Corp
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,.
FDA Form 483 Warning Letters How to Handle, Form, Example » Pharmaguddu
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. During an inspection, ora investigators may observe conditions they deem to be objectionable.
(PDF) FDA Form 483 (PDF 8.44MB) DOKUMEN.TIPS
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,.
Difference Between Form FDA 483, Warning Letters and EIR PharmaJia
During an inspection, ora investigators may observe conditions they deem to be objectionable. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
How to Respond FDA Form 483 and Warning Letters Know its differences
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,.
FDA Form 483 Warning Letters How to Handle, Form, Example » Pharmaguddu
During an inspection, ora investigators may observe conditions they deem to be objectionable. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
483 Inspection Observation Responses Customs & International Trade
An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
What’s the Difference? FDA Form 483 Observations and Warning Letters
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable. An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,.
A Form Fda 483 Is Issued To Firm Management At The Conclusion Of An Inspection When An Investigator(S) Has Observed Conditions That In.
An fda form 483 highlights areas where a company needs to improve its manufacturing process to guarantee the proper preparation,. During an inspection, ora investigators may observe conditions they deem to be objectionable.