Fda 2253 Form
Fda 2253 Form - Transmittal of advertisements and promotional labeling for drugs and biologics for human use. March 31, 2024 see pra statement on. Form fda 2567 is equivalent to form fda 2253. This form may be outdated. Department of health and human services form approved:
This form may be outdated. March 31, 2024 see pra statement on. Form fda 2567 is equivalent to form fda 2253. Department of health and human services form approved: Transmittal of advertisements and promotional labeling for drugs and biologics for human use.
Form fda 2567 is equivalent to form fda 2253. Department of health and human services form approved: Transmittal of advertisements and promotional labeling for drugs and biologics for human use. This form may be outdated. March 31, 2024 see pra statement on.
FDA AdPromo Series 2253 Submissions in eCTD Format
Transmittal of advertisements and promotional labeling for drugs and biologics for human use. Form fda 2567 is equivalent to form fda 2253. March 31, 2024 see pra statement on. This form may be outdated. Department of health and human services form approved:
Images Of Fda Form
Department of health and human services form approved: This form may be outdated. Transmittal of advertisements and promotional labeling for drugs and biologics for human use. March 31, 2024 see pra statement on. Form fda 2567 is equivalent to form fda 2253.
Fda Form 2253 printable pdf download
Form fda 2567 is equivalent to form fda 2253. Department of health and human services form approved: Transmittal of advertisements and promotional labeling for drugs and biologics for human use. This form may be outdated. March 31, 2024 see pra statement on.
Form FDA 2253
Transmittal of advertisements and promotional labeling for drugs and biologics for human use. Department of health and human services form approved: This form may be outdated. March 31, 2024 see pra statement on. Form fda 2567 is equivalent to form fda 2253.
Deconstructing FDA 2253 Form Requirements and What it Means for the
This form may be outdated. Department of health and human services form approved: Transmittal of advertisements and promotional labeling for drugs and biologics for human use. March 31, 2024 see pra statement on. Form fda 2567 is equivalent to form fda 2253.
Form FDA2253 Fill Out, Sign Online and Download Fillable PDF
Form fda 2567 is equivalent to form fda 2253. Transmittal of advertisements and promotional labeling for drugs and biologics for human use. This form may be outdated. Department of health and human services form approved: March 31, 2024 see pra statement on.
Fillable Online fda Ayerst Laboratories (Wyeth) on Form FDA 2253, that
March 31, 2024 see pra statement on. Form fda 2567 is equivalent to form fda 2253. This form may be outdated. Transmittal of advertisements and promotional labeling for drugs and biologics for human use. Department of health and human services form approved:
FDA 2253 Form Requirements and the Content Lifecycle The Leader in
Form fda 2567 is equivalent to form fda 2253. Transmittal of advertisements and promotional labeling for drugs and biologics for human use. This form may be outdated. Department of health and human services form approved: March 31, 2024 see pra statement on.
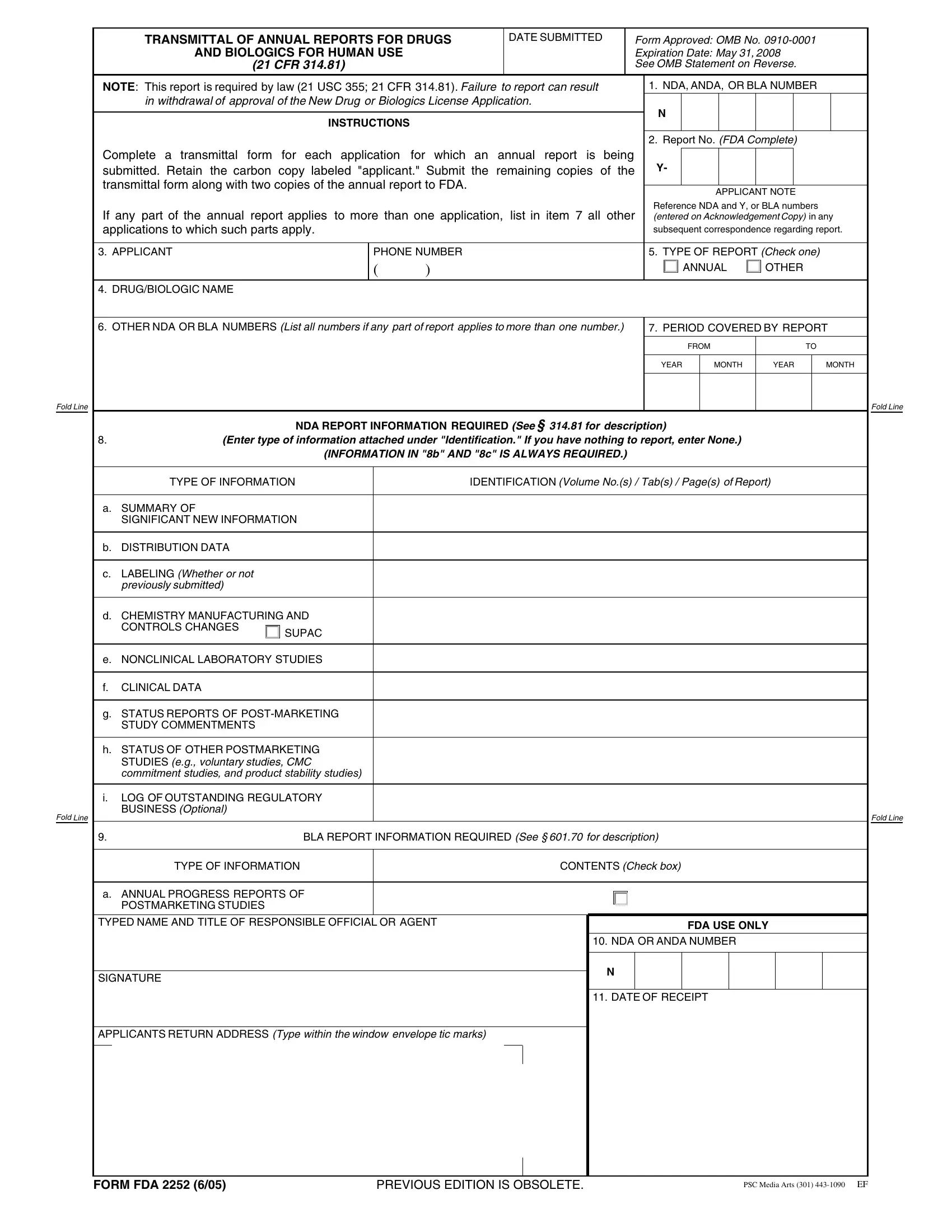
Form Fda 2252 ≡ Fill Out Printable PDF Forms Online
Transmittal of advertisements and promotional labeling for drugs and biologics for human use. Department of health and human services form approved: March 31, 2024 see pra statement on. Form fda 2567 is equivalent to form fda 2253. This form may be outdated.
Instructions For Submitting Form Fda2253 printable pdf download
Transmittal of advertisements and promotional labeling for drugs and biologics for human use. This form may be outdated. Form fda 2567 is equivalent to form fda 2253. Department of health and human services form approved: March 31, 2024 see pra statement on.
Transmittal Of Advertisements And Promotional Labeling For Drugs And Biologics For Human Use.
Form fda 2567 is equivalent to form fda 2253. March 31, 2024 see pra statement on. This form may be outdated. Department of health and human services form approved: