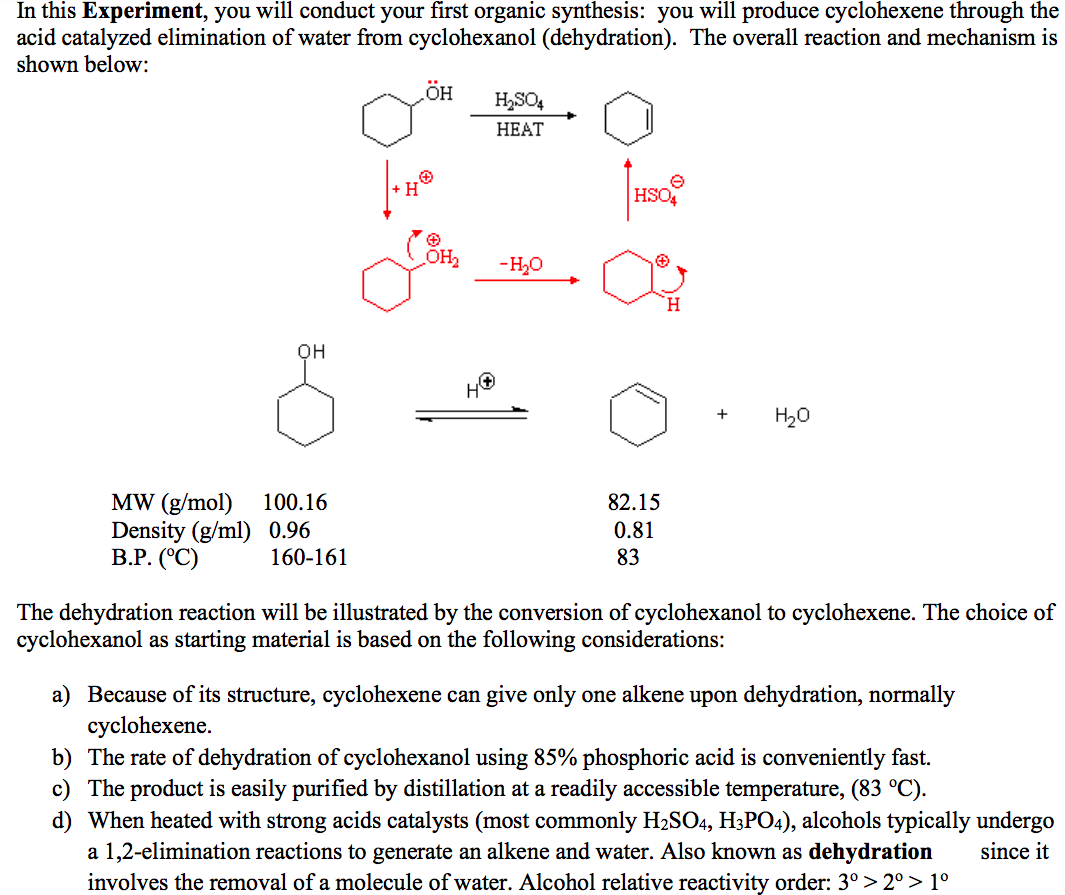
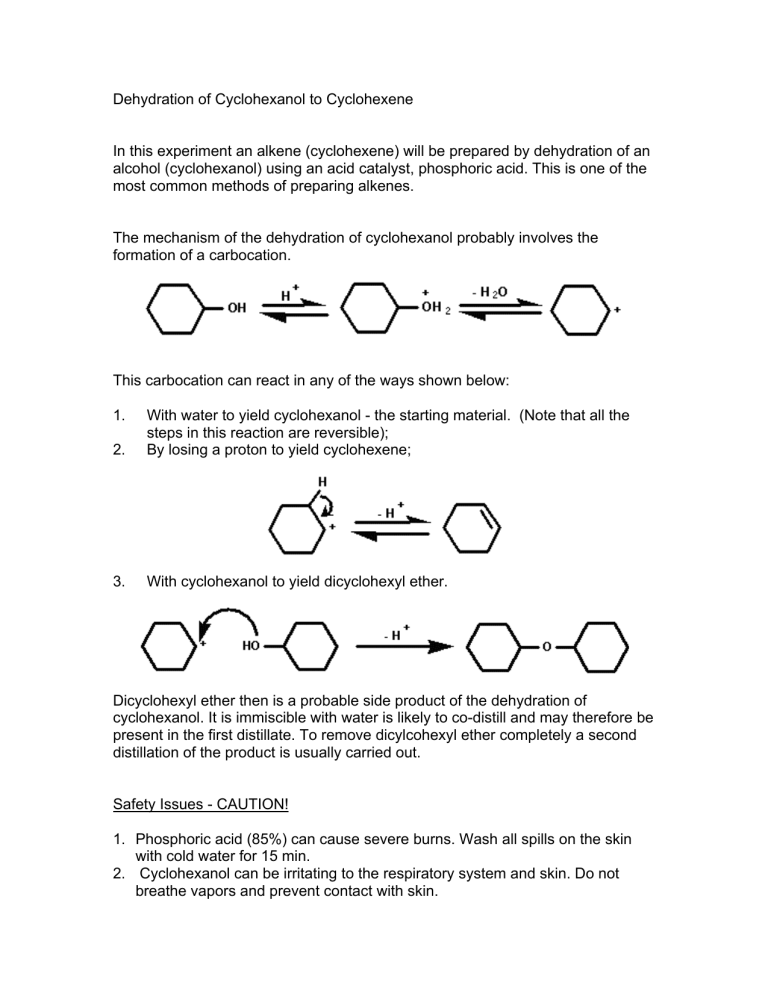
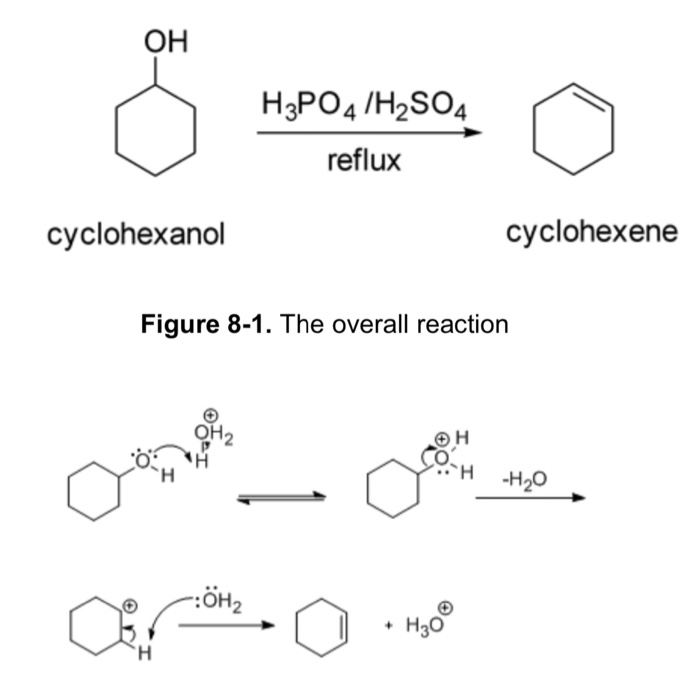
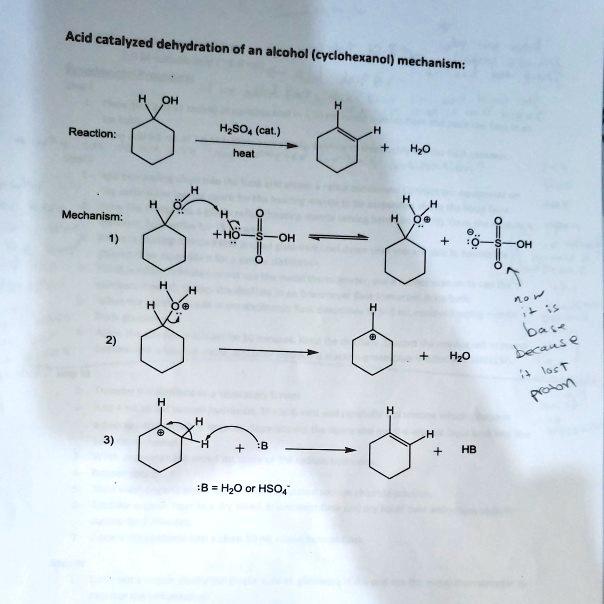
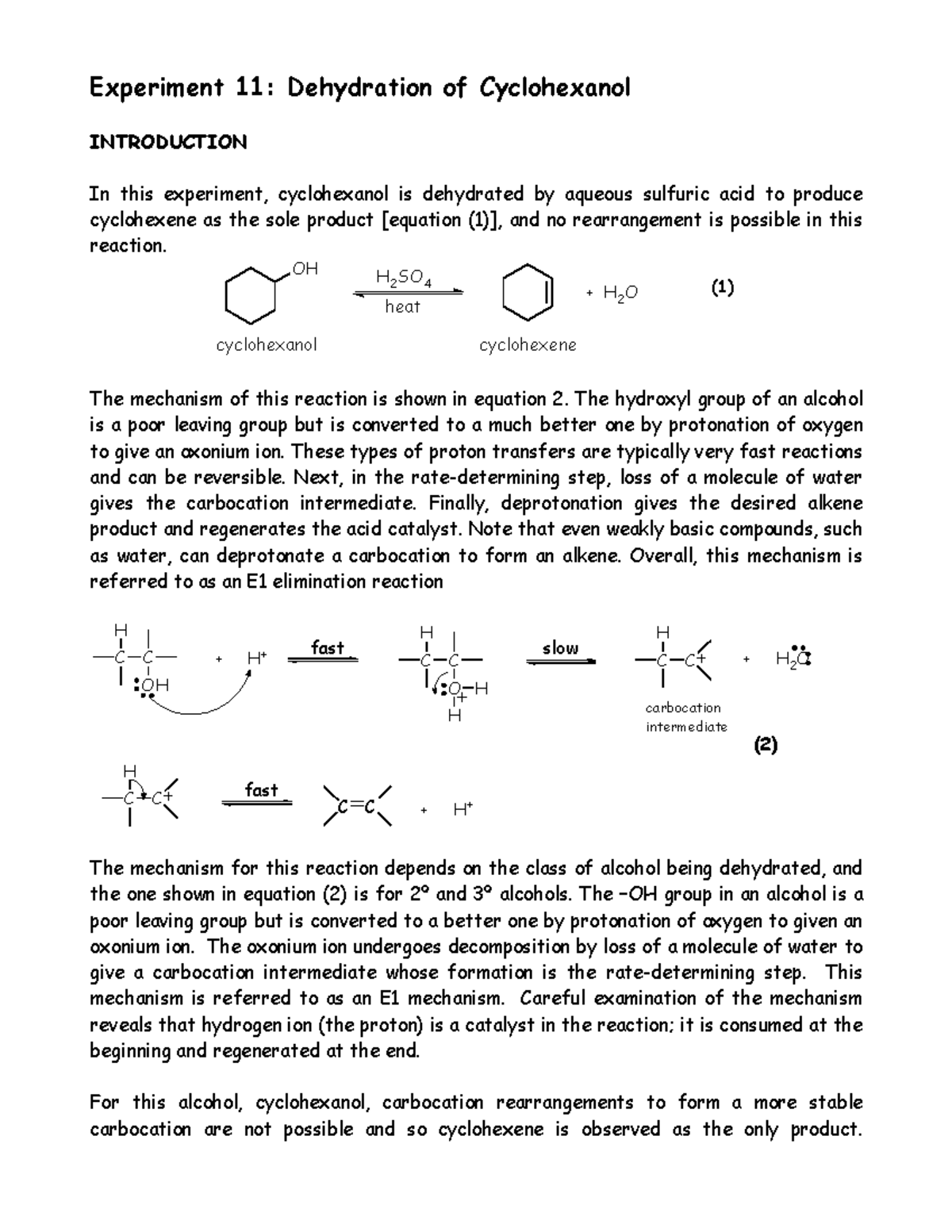
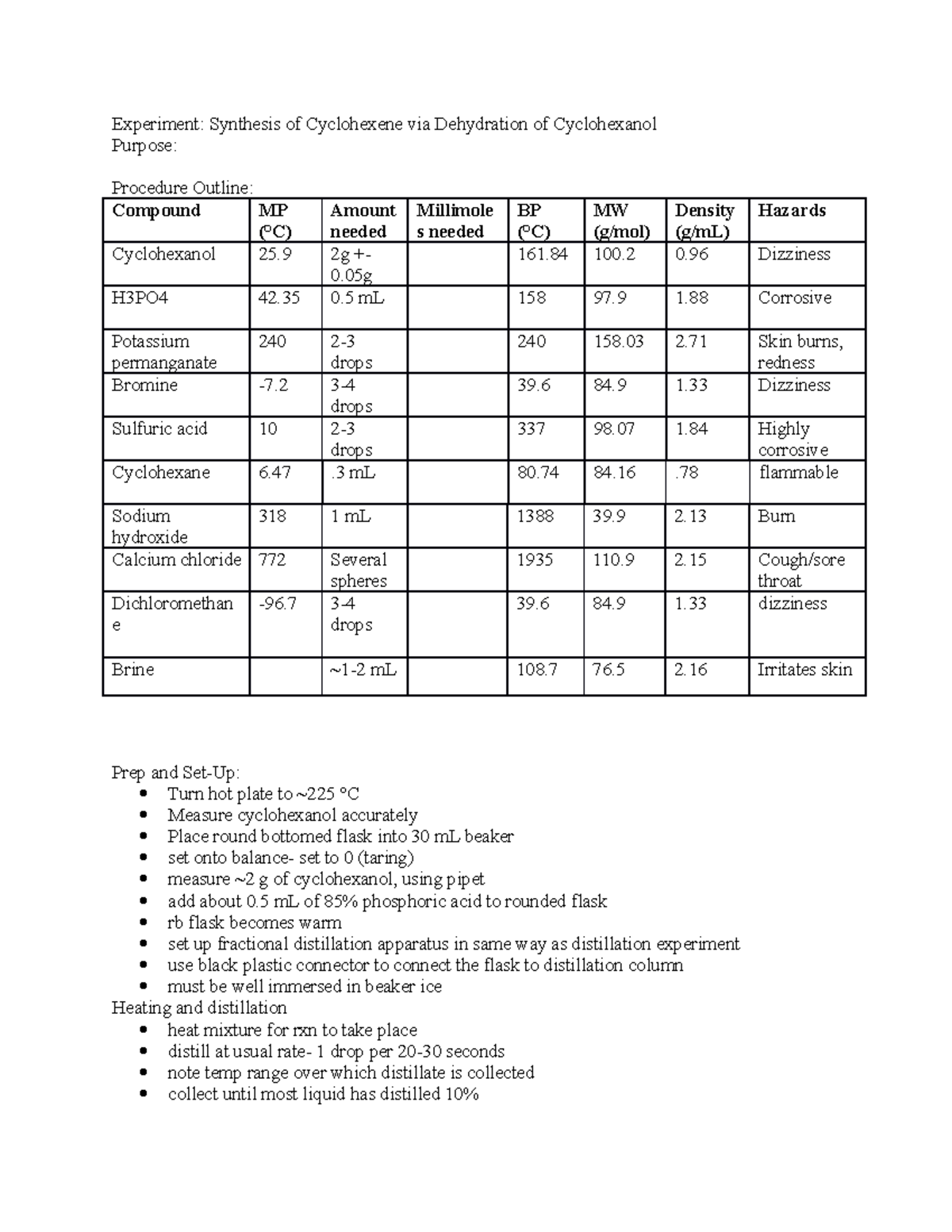
Dehydration Of Cyclohexanol To Cyclohexene
Dehydration Of Cyclohexanol To Cyclohexene - The cyclohexene product will be isolated by simple. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and. The dehydration of cyclohexanol to yield cyclohexene. Theoretically, in a dehydration reaction, one mole of alcohol.
Theoretically, in a dehydration reaction, one mole of alcohol. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The dehydration of cyclohexanol to yield cyclohexene. The cyclohexene product will be isolated by simple. This is a preparation commonly used to illustrate the formation and.
Theoretically, in a dehydration reaction, one mole of alcohol. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The dehydration of cyclohexanol to yield cyclohexene. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple.
Cyclohexanol Dehydration
From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. Theoretically, in a dehydration reaction, one mole of alcohol. The cyclohexene product will be isolated by simple. This is a preparation commonly used to illustrate the formation and. The dehydration of cyclohexanol to yield cyclohexene.
[Solved] Synthesis of Cyclohexene by Dehydration of Cyclohexanol There
From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The dehydration of cyclohexanol to yield cyclohexene. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple.
Synthesis of Cyclohexene via Dehydration of Cyclohexanol lab
From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The cyclohexene product will be isolated by simple. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. The dehydration of cyclohexanol to yield cyclohexene.
Cyclohexene from Cyclohexanol Dehydration of alcohol YouTube
The cyclohexene product will be isolated by simple. The dehydration of cyclohexanol to yield cyclohexene. This is a preparation commonly used to illustrate the formation and. Theoretically, in a dehydration reaction, one mole of alcohol. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced.
Cyclohexanol Dehydration
From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. The dehydration of cyclohexanol to yield cyclohexene. The cyclohexene product will be isolated by simple.
Solved (iv). What type of reaction is the conversion of
From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and. The dehydration of cyclohexanol to yield cyclohexene. The cyclohexene product will be isolated by simple. Theoretically, in a dehydration reaction, one mole of alcohol.
Cyclohexanol Dehydration
This is a preparation commonly used to illustrate the formation and. The dehydration of cyclohexanol to yield cyclohexene. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. Theoretically, in a dehydration reaction, one mole of alcohol. The cyclohexene product will be isolated by simple.
dehydration of cyclohexane Experiment 11 Dehydration of Cyclohexanol
The dehydration of cyclohexanol to yield cyclohexene. Theoretically, in a dehydration reaction, one mole of alcohol. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple.
Practical skills assessment video the dehydration of cyclohexanol to
Theoretically, in a dehydration reaction, one mole of alcohol. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple. The dehydration of cyclohexanol to yield cyclohexene.
Pre lab 6 Synthesis of Cyclohexene via Dehydration of Cyclohexanol
This is a preparation commonly used to illustrate the formation and. The dehydration of cyclohexanol to yield cyclohexene. Theoretically, in a dehydration reaction, one mole of alcohol. The cyclohexene product will be isolated by simple. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced.
The Dehydration Of Cyclohexanol To Yield Cyclohexene.
Theoretically, in a dehydration reaction, one mole of alcohol. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple.