Case Report Form
Case Report Form - What is a case report form? This article will discuss different case report forms and show. A crf is a set of documents that collects data and information from a clinical trial. [1] the case report form is the tool used by the sponsor of the clinical trial to collect. A case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. It enables efficient and complete. A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. The crf is used by the study sponsor to capture and retain. In a case report form, you can track the unique changes of each research subject as the clinical trial progresses.
A crf is a set of documents that collects data and information from a clinical trial. This article will discuss different case report forms and show. [1] the case report form is the tool used by the sponsor of the clinical trial to collect. The crf is used by the study sponsor to capture and retain. A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. A case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. It enables efficient and complete. What is a case report form? In a case report form, you can track the unique changes of each research subject as the clinical trial progresses.
A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. A crf is a set of documents that collects data and information from a clinical trial. It enables efficient and complete. What is a case report form? In a case report form, you can track the unique changes of each research subject as the clinical trial progresses. This article will discuss different case report forms and show. [1] the case report form is the tool used by the sponsor of the clinical trial to collect. A case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. The crf is used by the study sponsor to capture and retain.
Case Report Form Semantic Scholar
[1] the case report form is the tool used by the sponsor of the clinical trial to collect. It enables efficient and complete. A case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. The crf is used by the study sponsor to.
What Is a Case Report Form? [ Importance, Tips, Samples ]
A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. The crf is used by the study sponsor to capture and retain. In a case report form, you can track the unique changes of each research subject as the clinical trial progresses. This article will discuss different case report forms and show..
FREE 15+ Case Report Forms in PDF MS Word
A crf is a set of documents that collects data and information from a clinical trial. It enables efficient and complete. The crf is used by the study sponsor to capture and retain. What is a case report form? A case report form (crf) is an electronic or paper document which is used in a clinical trial to record the.
Case Report Form Template Professional Template
In a case report form, you can track the unique changes of each research subject as the clinical trial progresses. A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. What is a case report form? It enables efficient and complete. This article will discuss different case report forms and show.
Case Report Form Template Sampletemplate.my.id
What is a case report form? This article will discuss different case report forms and show. A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. A crf is a set of documents that collects data and information from a clinical trial. A case report form (crf) is an electronic or paper.
Case Report Form RIAT Support Center
A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. A case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. This article will discuss different case report forms and show. What is a case.
Case Report Form ≡ Fill Out Printable PDF Forms Online
What is a case report form? A crf is a set of documents that collects data and information from a clinical trial. The crf is used by the study sponsor to capture and retain. This article will discuss different case report forms and show. A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical.
FREE 15+ Case Report Forms in PDF MS Word
[1] the case report form is the tool used by the sponsor of the clinical trial to collect. This article will discuss different case report forms and show. A crf is a set of documents that collects data and information from a clinical trial. In a case report form, you can track the unique changes of each research subject as.
FREE 15+ Case Report Forms in PDF MS Word
This article will discuss different case report forms and show. In a case report form, you can track the unique changes of each research subject as the clinical trial progresses. A case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. It enables.
What Is a Case Report Form? [ Importance, Tips, Samples ]
[1] the case report form is the tool used by the sponsor of the clinical trial to collect. The crf is used by the study sponsor to capture and retain. A crf is a set of documents that collects data and information from a clinical trial. What is a case report form? A case report form (crf) is an electronic.
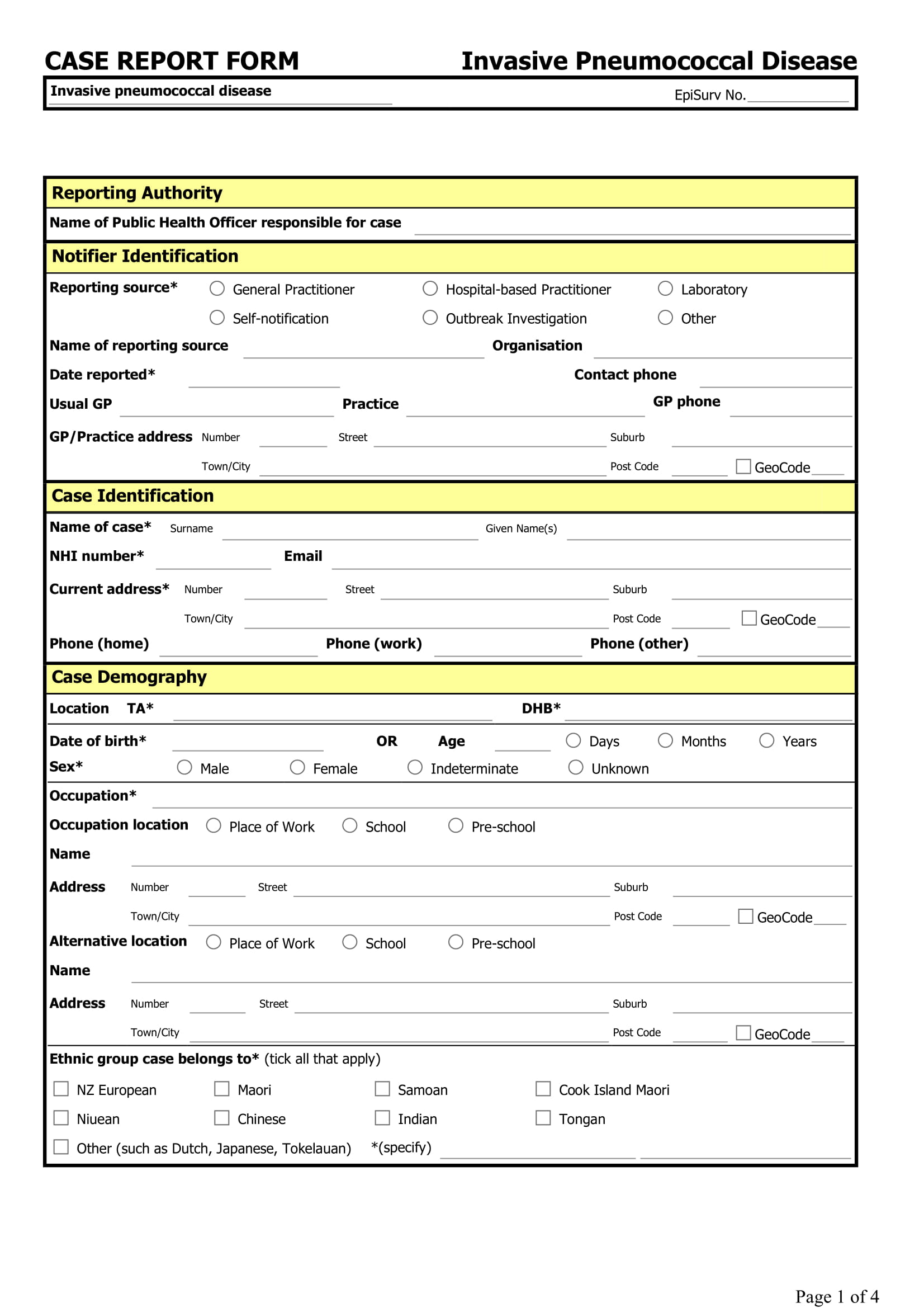
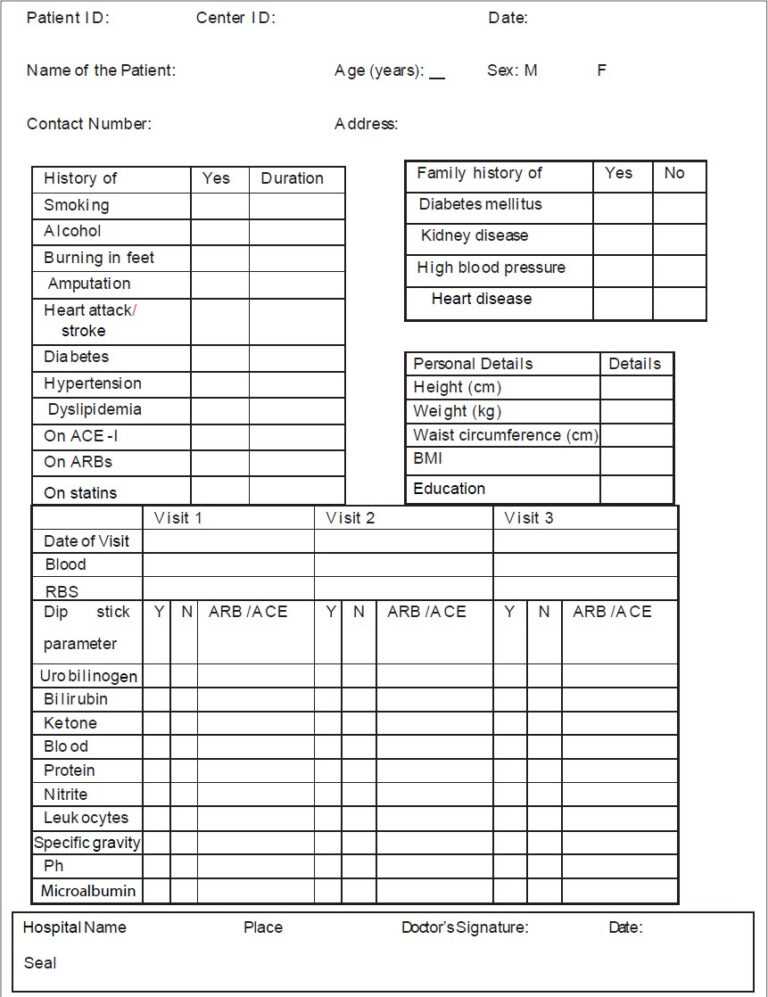
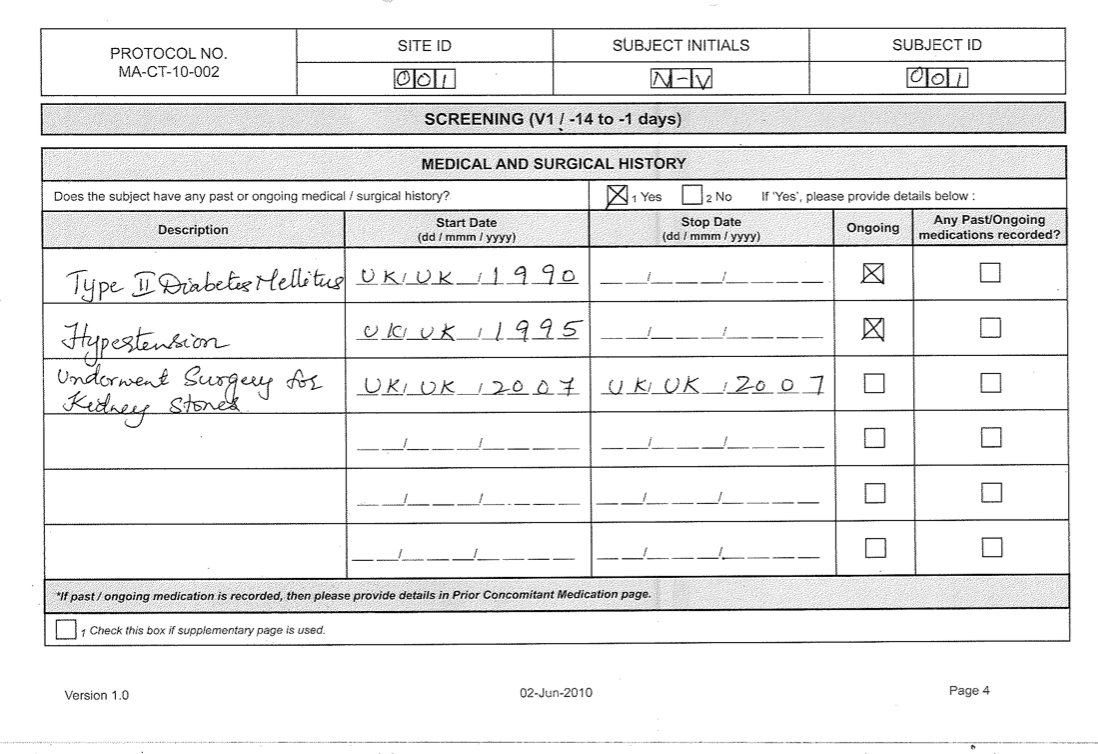
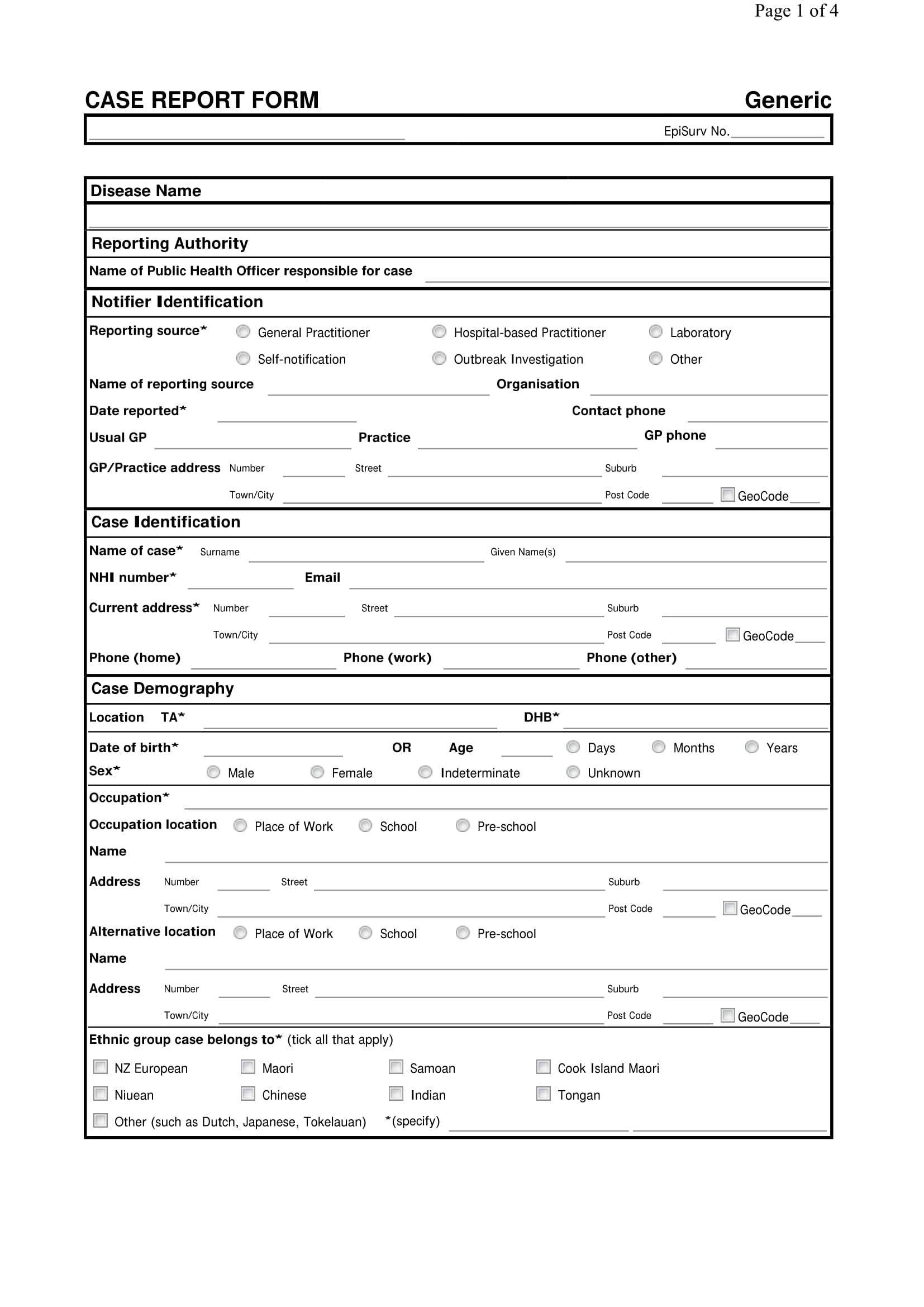
A Case Report Form (Crf) Is An Electronic Or Paper Document Which Is Used In A Clinical Trial To Record The Protocol And Required Information About Each Participant.
[1] the case report form is the tool used by the sponsor of the clinical trial to collect. The crf is used by the study sponsor to capture and retain. A crf is a set of documents that collects data and information from a clinical trial. What is a case report form?
In A Case Report Form, You Can Track The Unique Changes Of Each Research Subject As The Clinical Trial Progresses.
A case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. It enables efficient and complete. This article will discuss different case report forms and show.

![What Is a Case Report Form? [ Importance, Tips, Samples ]](https://images.sampleforms.com/wp-content/uploads/2018/02/Confidential-Case-Report-Form-in-PDF-1.jpg)



![What Is a Case Report Form? [ Importance, Tips, Samples ]](https://images.sampleforms.com/wp-content/uploads/2018/02/Mortality-Case-Report-Form-Sample-1.jpg)