Can Propane Form Isomers
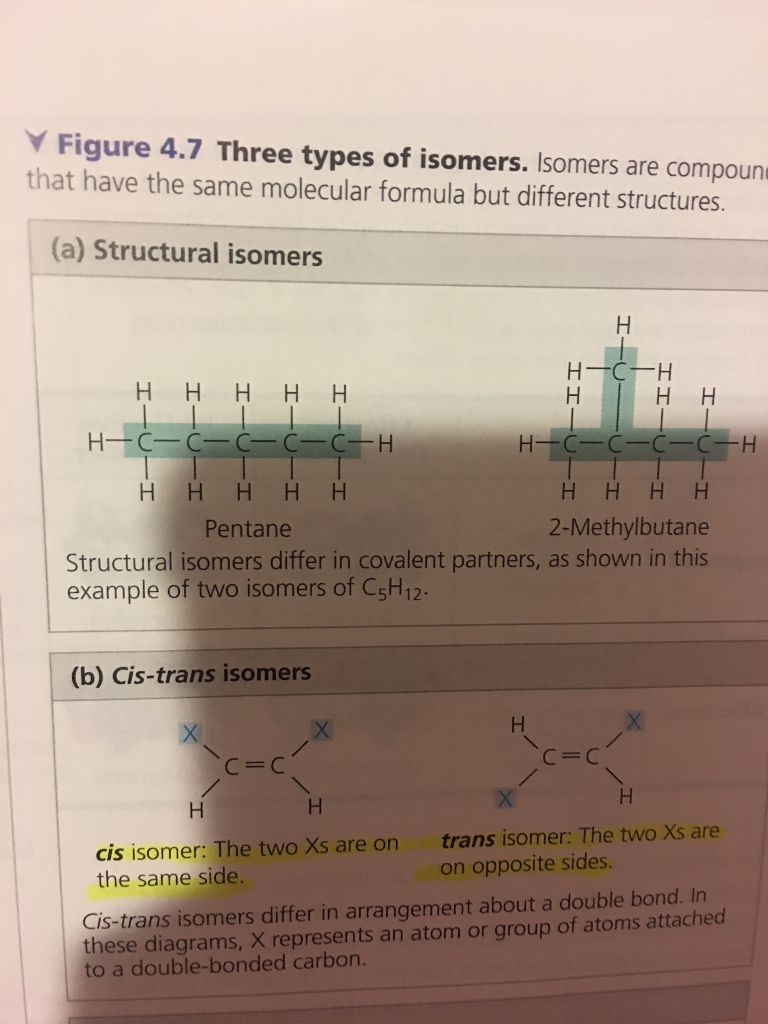
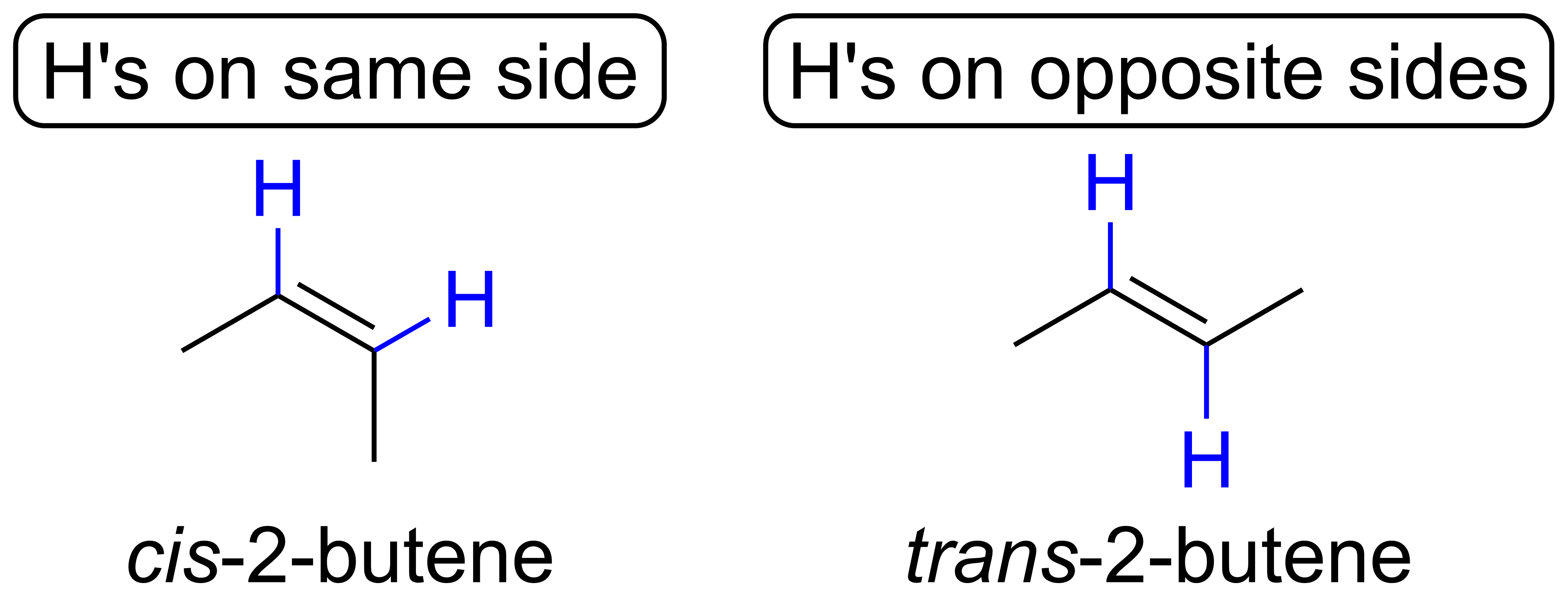
Can Propane Form Isomers - In alkanes, isomerism arises when a particular compound. Structural isomers are compounds with the same molecular formula but different. Find out why propane cannot have any isomers due to its. As there is no double bond in. Propane (c3h8) does not have any structural isomers. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Learn the definition and examples of structural isomerism in organic chemistry. For isomers to exist, there should be a minimum of four carbons for branching to take place. However iff there is hetero atom or any atom.
Find out why propane cannot have any isomers due to its. However iff there is hetero atom or any atom. In alkanes, isomerism arises when a particular compound. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. As there is no double bond in. Learn the definition and examples of structural isomerism in organic chemistry. Propane (c3h8) does not have any structural isomers. For isomers to exist, there should be a minimum of four carbons for branching to take place. Structural isomers are compounds with the same molecular formula but different.
Propane (c3h8) does not have any structural isomers. In alkanes, isomerism arises when a particular compound. Learn the definition and examples of structural isomerism in organic chemistry. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Find out why propane cannot have any isomers due to its. As there is no double bond in. For isomers to exist, there should be a minimum of four carbons for branching to take place. However iff there is hetero atom or any atom. Structural isomers are compounds with the same molecular formula but different.
Una Breve Guida per Tipi di Isomeria in Chimica Organica Interesse
Propane (c3h8) does not have any structural isomers. As there is no double bond in. However iff there is hetero atom or any atom. For isomers to exist, there should be a minimum of four carbons for branching to take place. Find out why propane cannot have any isomers due to its.
Solved See figures 4.5a and 4.7. Can propane (C3H8) form
Find out why propane cannot have any isomers due to its. Learn the definition and examples of structural isomerism in organic chemistry. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Structural isomers are compounds with the same molecular formula but different. Propane (c3h8) does not have any structural isomers.
Chapter 4 Carbon and its Compounds NCERT Solutions for Class 10
As there is no double bond in. Learn the definition and examples of structural isomerism in organic chemistry. Structural isomers are compounds with the same molecular formula but different. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Find out why propane cannot have any isomers due to its.
How many structural isomers can you draw pentane?
Find out why propane cannot have any isomers due to its. Propane (c3h8) does not have any structural isomers. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Learn the definition and examples of structural isomerism in organic chemistry. As there is no double bond in.
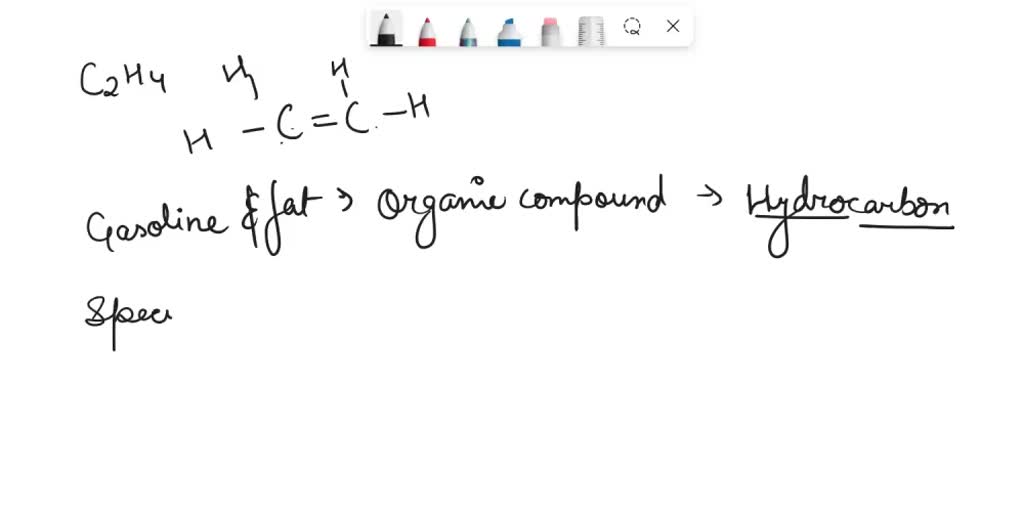
SOLVED 1) Draw structural formula for C2H4. 2) How are gasoline and
Structural isomers are compounds with the same molecular formula but different. Find out why propane cannot have any isomers due to its. As there is no double bond in. For isomers to exist, there should be a minimum of four carbons for branching to take place. Learn the definition and examples of structural isomerism in organic chemistry.
Isomers vs Conformers CHEM123 chirp
For isomers to exist, there should be a minimum of four carbons for branching to take place. In alkanes, isomerism arises when a particular compound. Learn the definition and examples of structural isomerism in organic chemistry. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Find out why propane cannot have any.
What Are The Isomers Of Propanol? How Are They Determined?
Find out why propane cannot have any isomers due to its. However iff there is hetero atom or any atom. Learn the definition and examples of structural isomerism in organic chemistry. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Propane (c3h8) does not have any structural isomers.
Why are there no isomers of propane?
In alkanes, isomerism arises when a particular compound. Propane (c3h8) does not have any structural isomers. Learn the definition and examples of structural isomerism in organic chemistry. For isomers to exist, there should be a minimum of four carbons for branching to take place. However iff there is hetero atom or any atom.
CHEM Alkanes and Alkenes chemistry organic chemistr...
Propane (c3h8) does not have any structural isomers. However iff there is hetero atom or any atom. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Find out why propane cannot have any isomers due to its. In alkanes, isomerism arises when a particular compound.
SOLVED What are isomers? Write the isomers of butane and give their
Structural isomers are compounds with the same molecular formula but different. In alkanes, isomerism arises when a particular compound. For isomers to exist, there should be a minimum of four carbons for branching to take place. Learn the definition and examples of structural isomerism in organic chemistry. Find out why propane cannot have any isomers due to its.
Find Out Why Propane Cannot Have Any Isomers Due To Its.
Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Structural isomers are compounds with the same molecular formula but different. However iff there is hetero atom or any atom. Learn the definition and examples of structural isomerism in organic chemistry.
As There Is No Double Bond In.
Propane (c3h8) does not have any structural isomers. In alkanes, isomerism arises when a particular compound. For isomers to exist, there should be a minimum of four carbons for branching to take place.