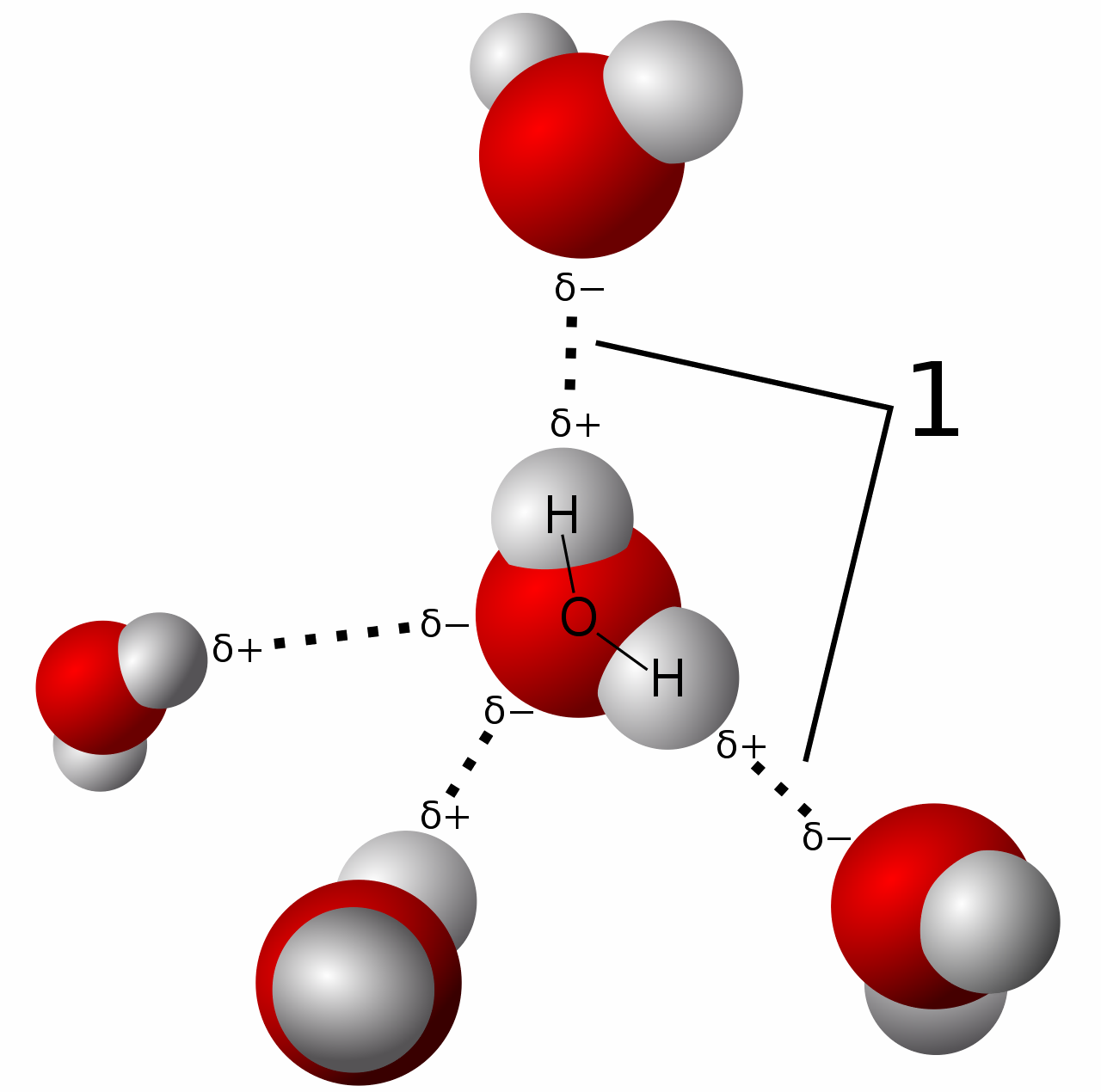
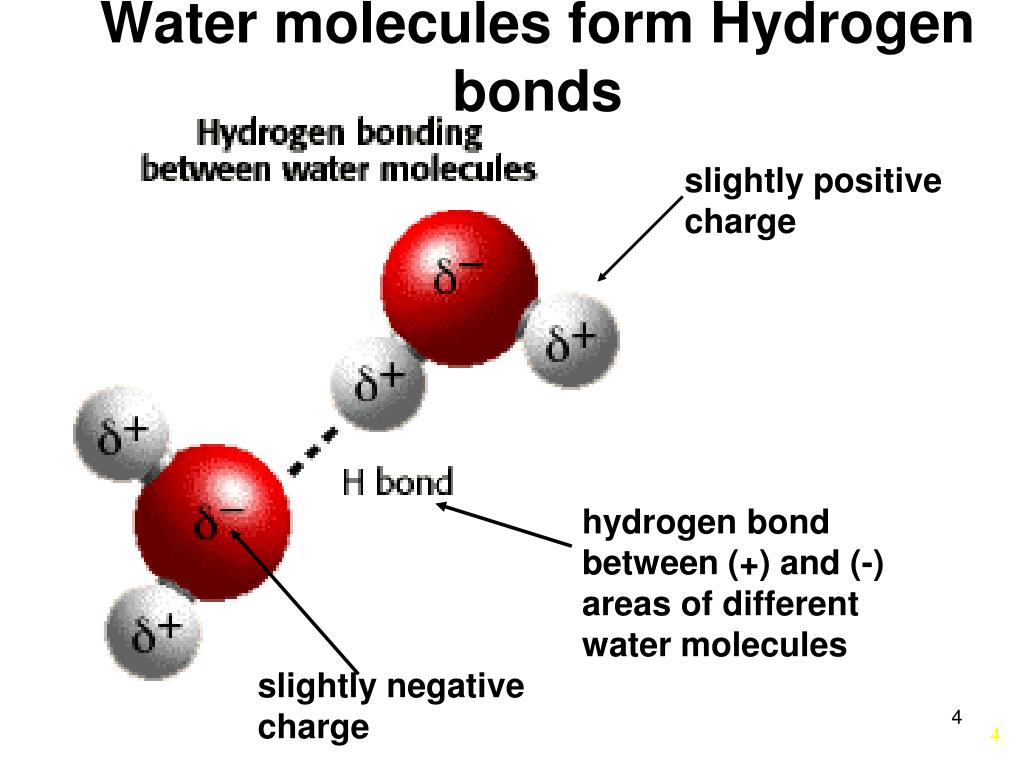
Bonds That Form Between Water Molecules Are Called
Bonds That Form Between Water Molecules Are Called - In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. Which of the following properties of. The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts.
Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. Which of the following properties of. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules.
The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. Which of the following properties of. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules.
IB DP Biology SL复习笔记2.1.3 Hydrogen Bonds翰林国际教育
But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. Which of the following properties of. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties.
Hydrogen Bonding Is The Effect Of Water Molecules Attracted
But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. Which of the following properties of..
110 Properties of Water Lab Makeup) Professor St. John's
Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. Which of the following properties of. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. The.
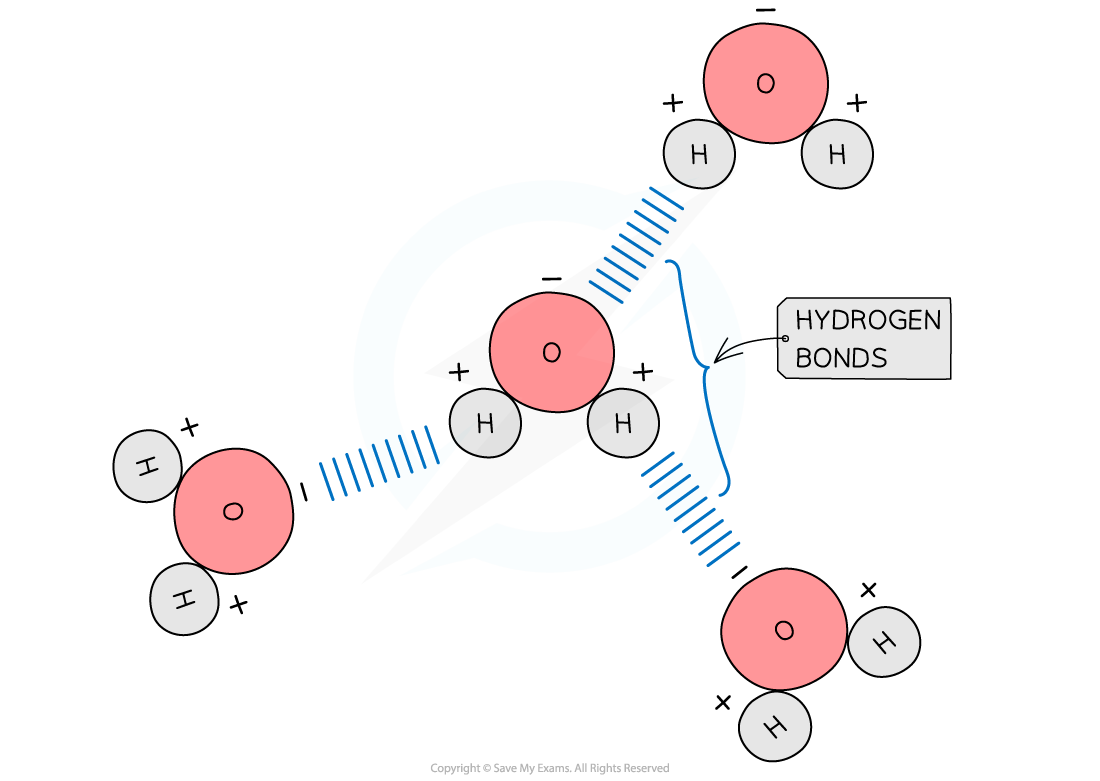
The diagram shows hydrogen bonds between water molecules. Label the
Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. Which of the following properties of. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. In.
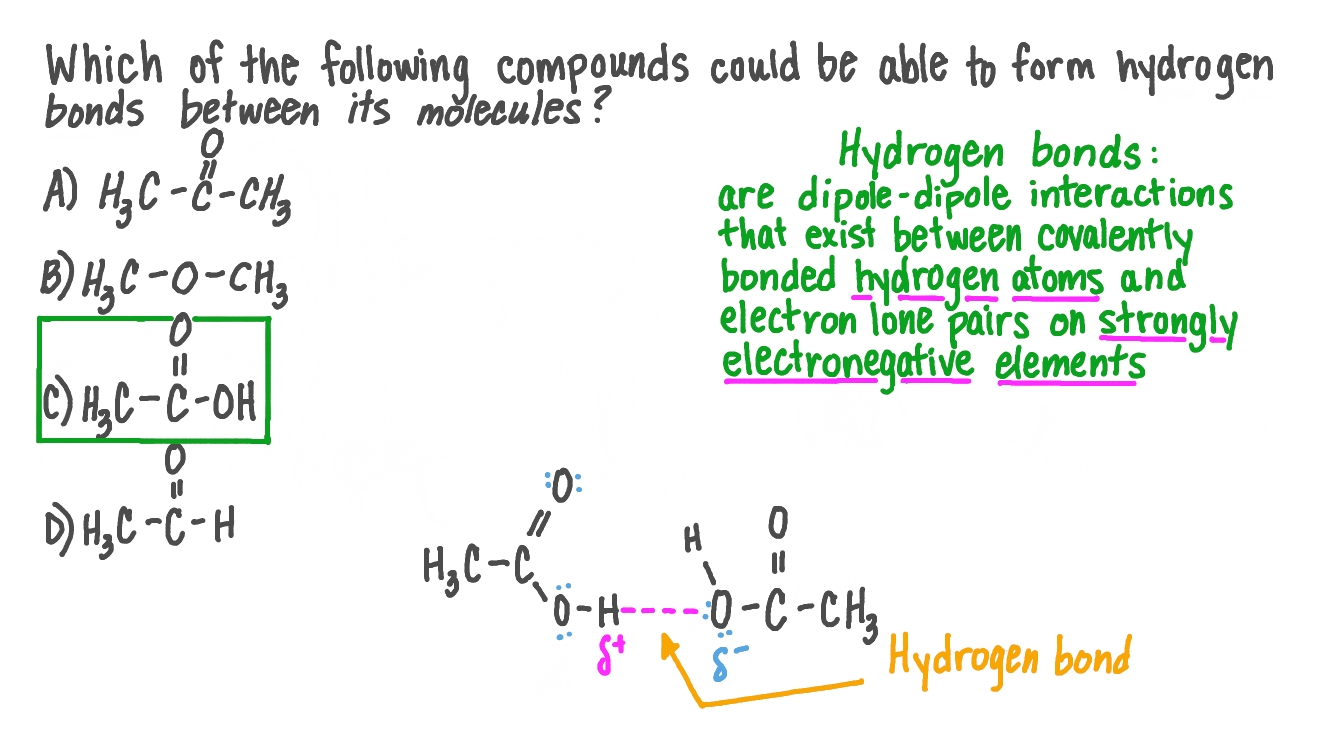
Question Video Identifying Which Compound Could Form Hydrogen Bonds
Which of the following properties of. Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. In.
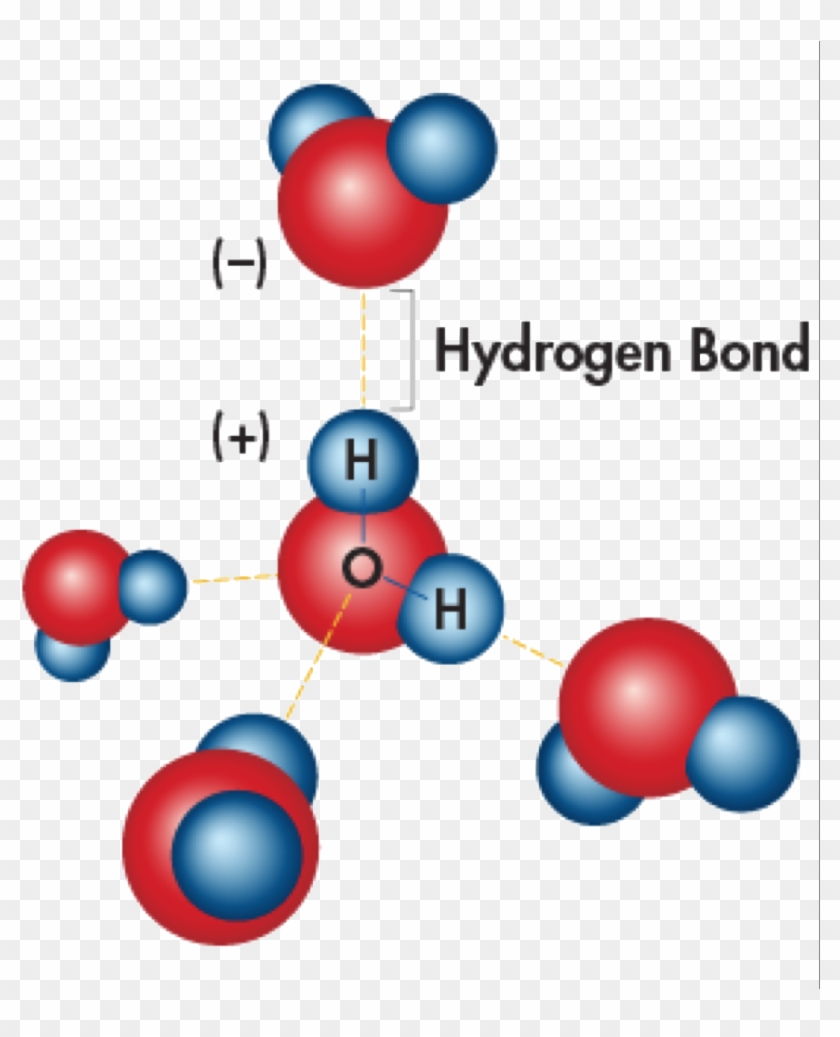
learning through art water molecules and hydrogen bonding
In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. But the intermolecular.
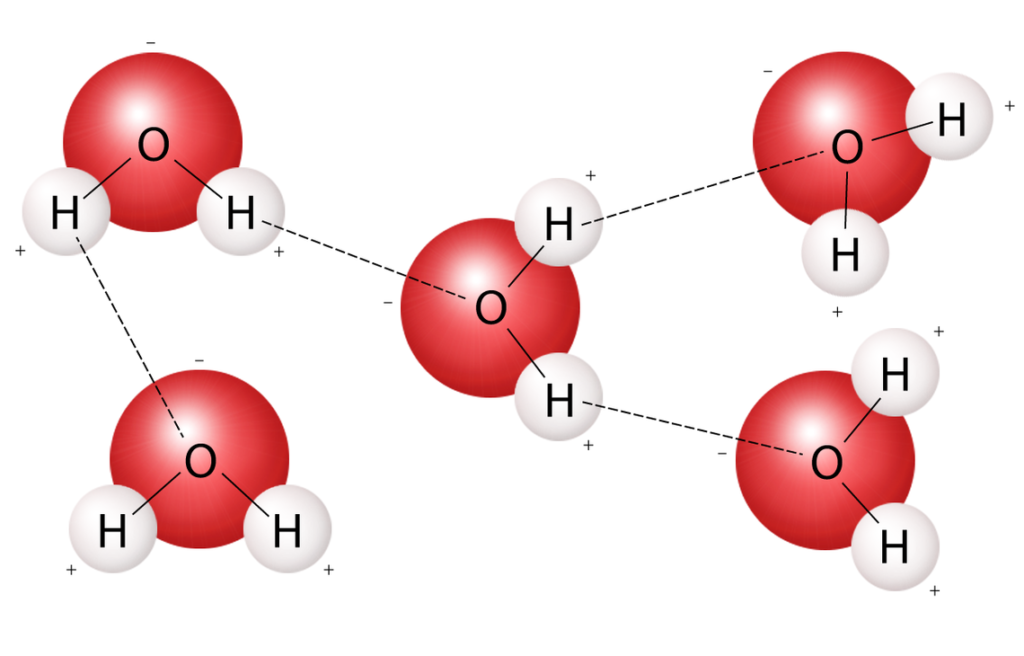
Dipoles Of Water Molecules ALevel Biology Revision Notes
The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. Which of the following properties of. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding..
Biomolecules flashcards 1 How do hydrogen bonds form between water
But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. Which of the following properties of. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. A drop of.
PPT Water Chemistry & Properties of Water PowerPoint Presentation
A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. Hydrogen bonds form between adjacent water molecules.
How To Draw Hydrogen Bonding
Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. Which of the following properties of. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent.
Which Of The Following Properties Of.
But the intermolecular bonds, the bonds between water molecules, are the result of hydrogen bonding. Hydrogen bonds form between adjacent water molecules because the positively charged hydrogen end of one water molecule attracts. A drop of falling water is a group of water molecules held together by the hydrogen bonds between the molecules. The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water.