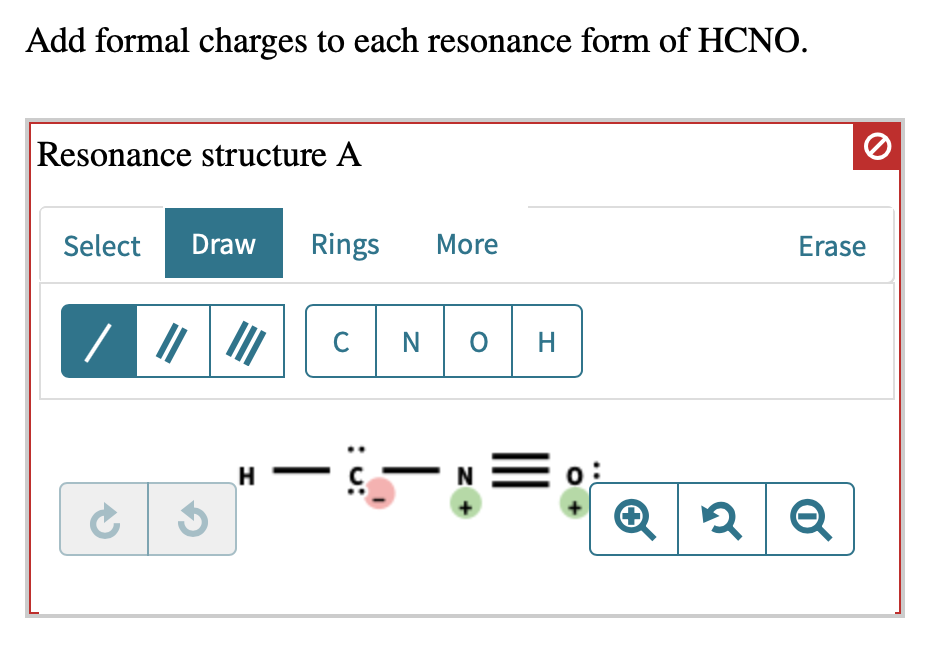
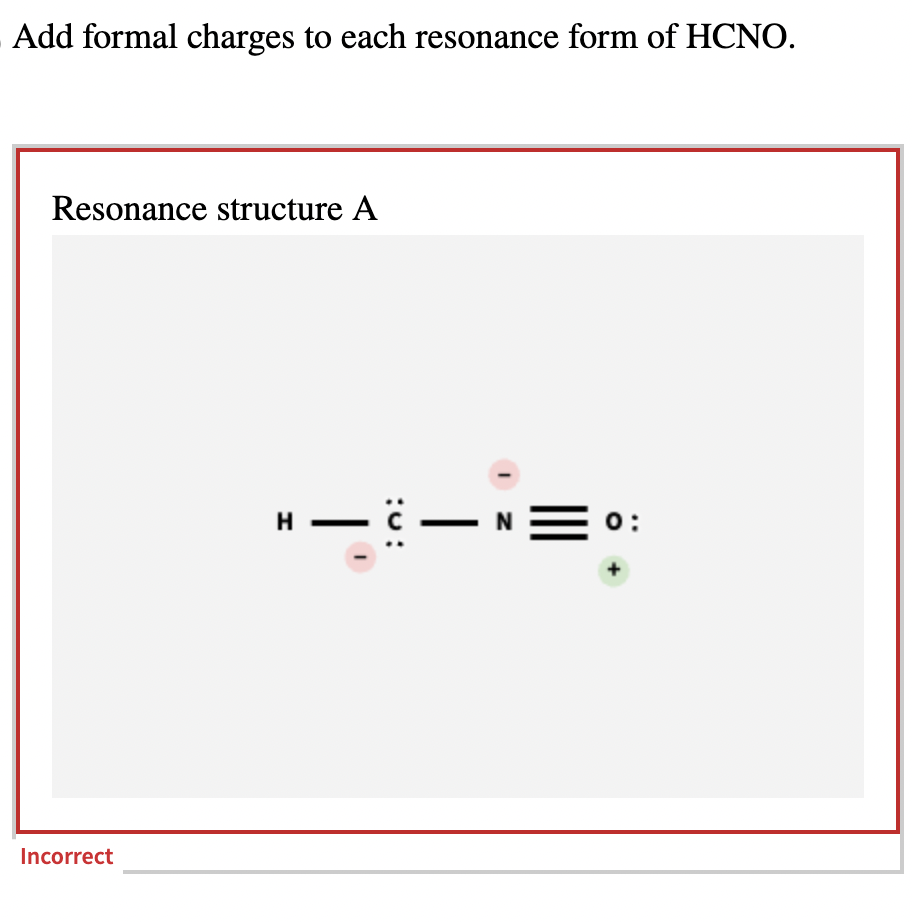
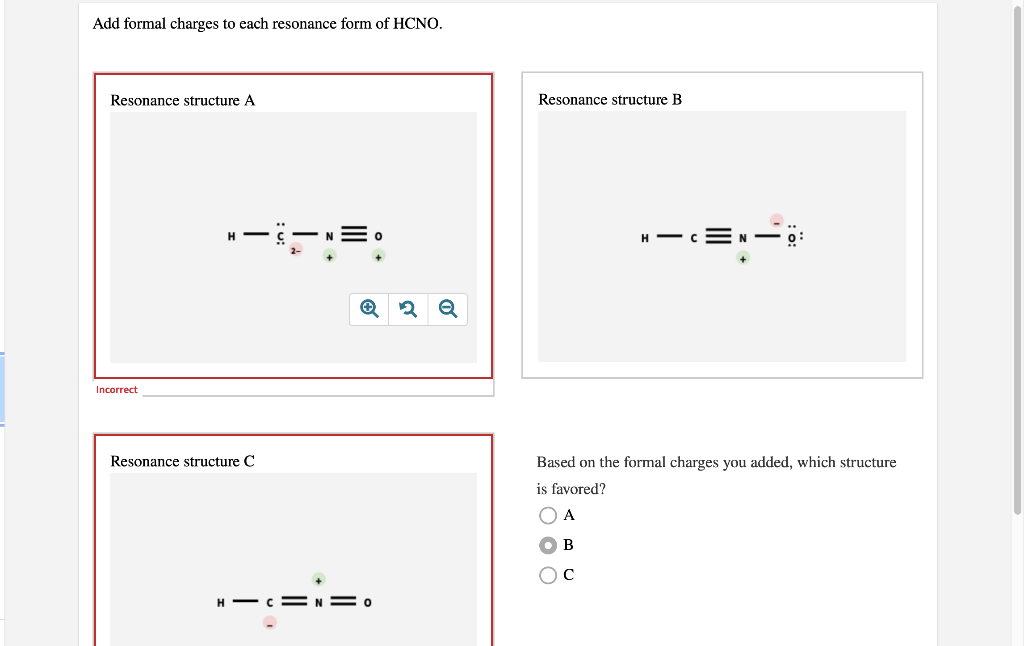
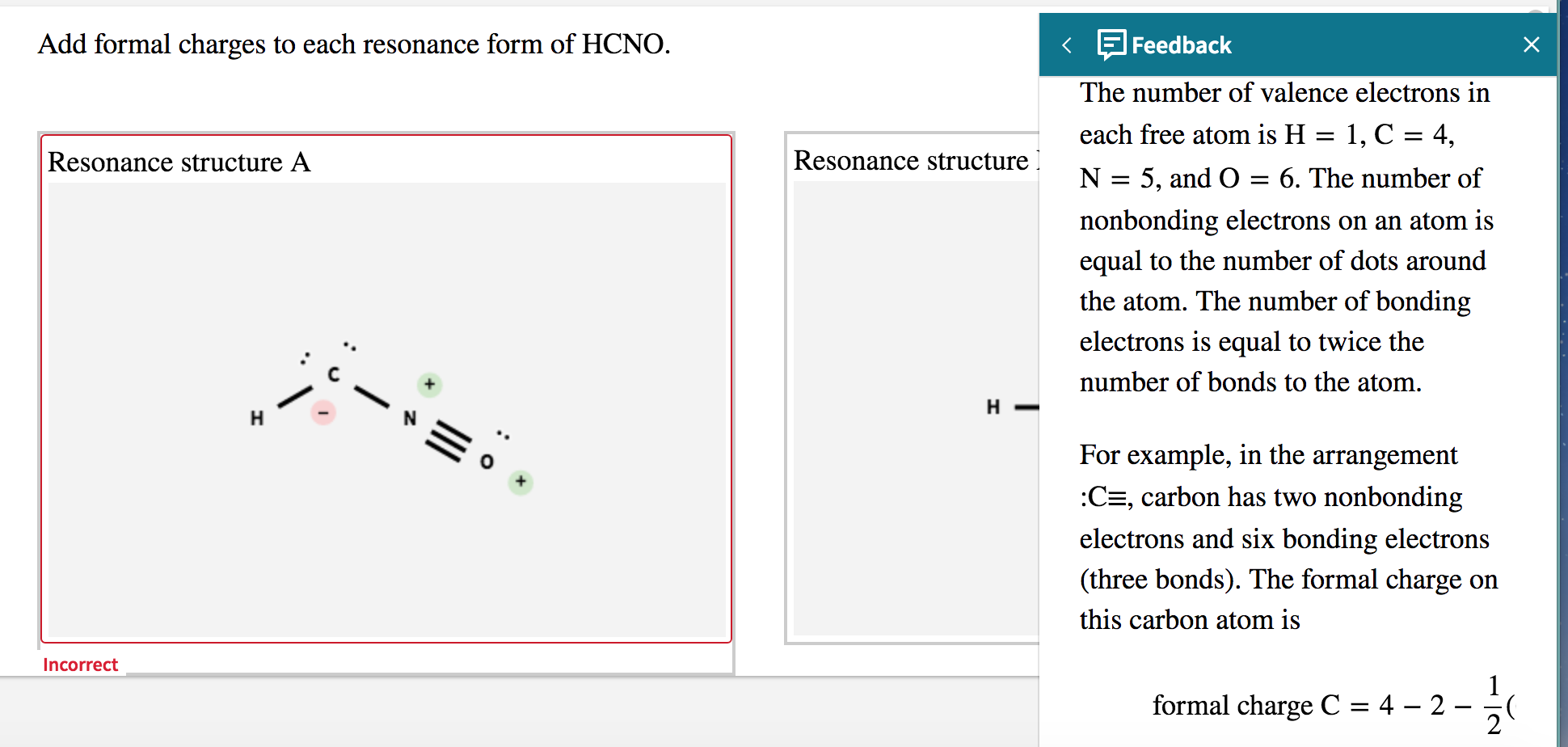
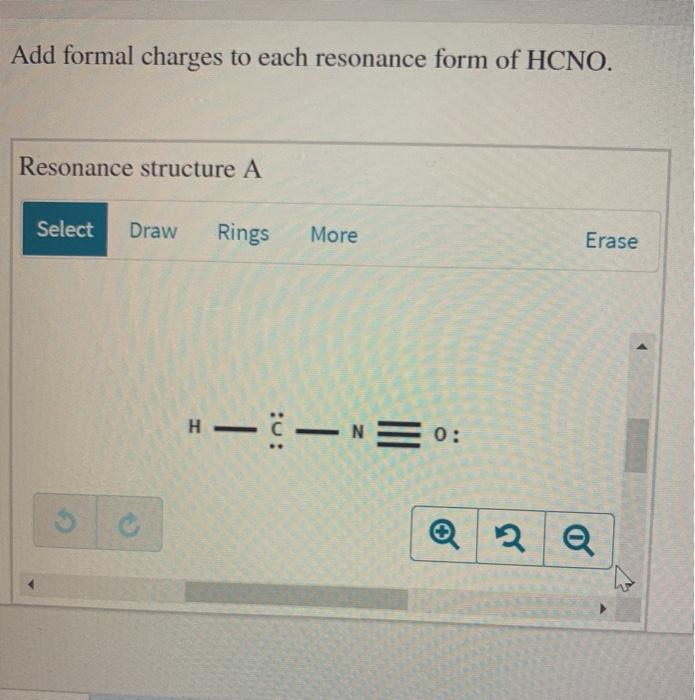
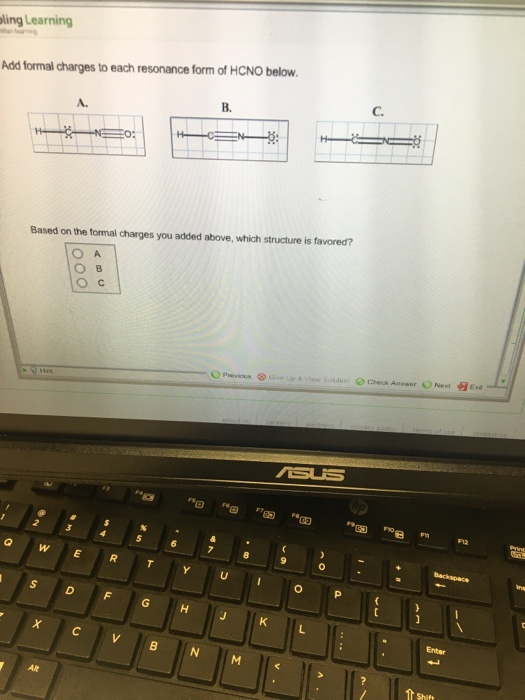
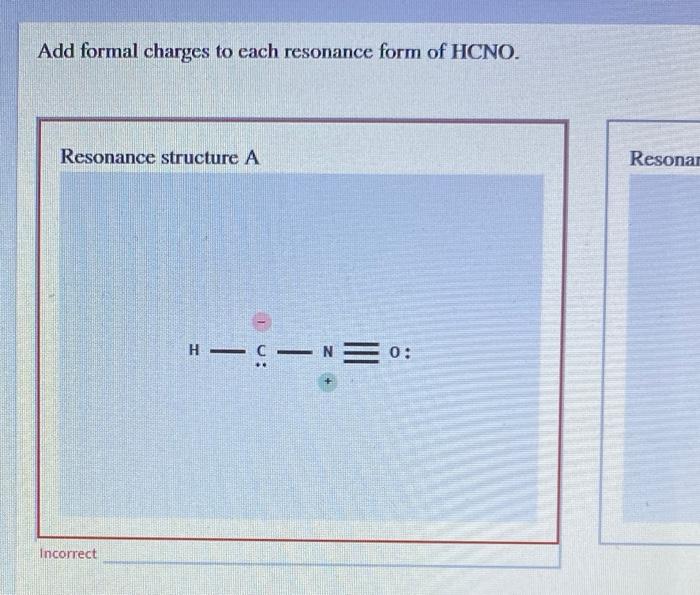
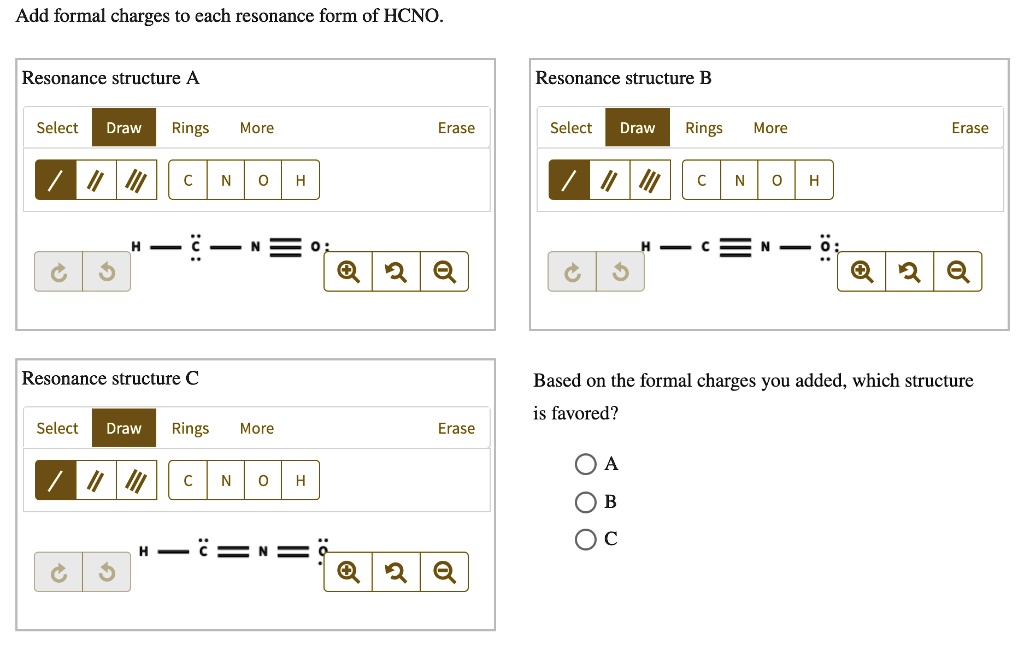
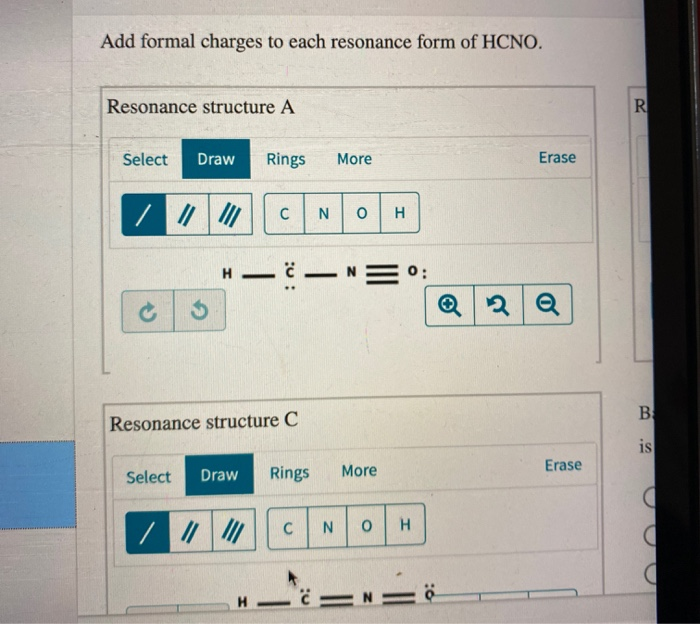
Add Formal Charges To Each Resonance Form Of Hcno
Add Formal Charges To Each Resonance Form Of Hcno - To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons.
A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons. To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form.
A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons.
Add Formal Charges To Each Resonance Form Of Hcno Below at tarcolbyblog
Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each.
Solved Add formal charges to each resonance form of HCNO.
A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons. To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each.
Add Formal Charges To Each Resonance Form Of Hcno Below at tarcolbyblog
To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of.
Solved Add formal charges to each resonance form of HCNO.
A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of.
Solved Add formal charges to each resonance form of HCNO. Х
Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons. To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable.
Solved Add formal charges to each resonance form of HCNO.
To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of.
Solved Add Formal Charges To Each Resonance Form Of HCNO
To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable.
Solved Add formal charges to each resonance form of HCNO.
To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of.
SOLVED Add formal charges to each resonance form of HCNO. Resonance
To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable one. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of.
Add Formal Charges To Each Resonance Form Of Hcno Below at tarcolbyblog
To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons. A forum post about adding formal charges to each resonance form of hcno and choosing the most stable.
A Forum Post About Adding Formal Charges To Each Resonance Form Of Hcno And Choosing The Most Stable One.
To determine the formal charges for each resonance form of hcno, we first need to draw the lewis structures for each form. Formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons.