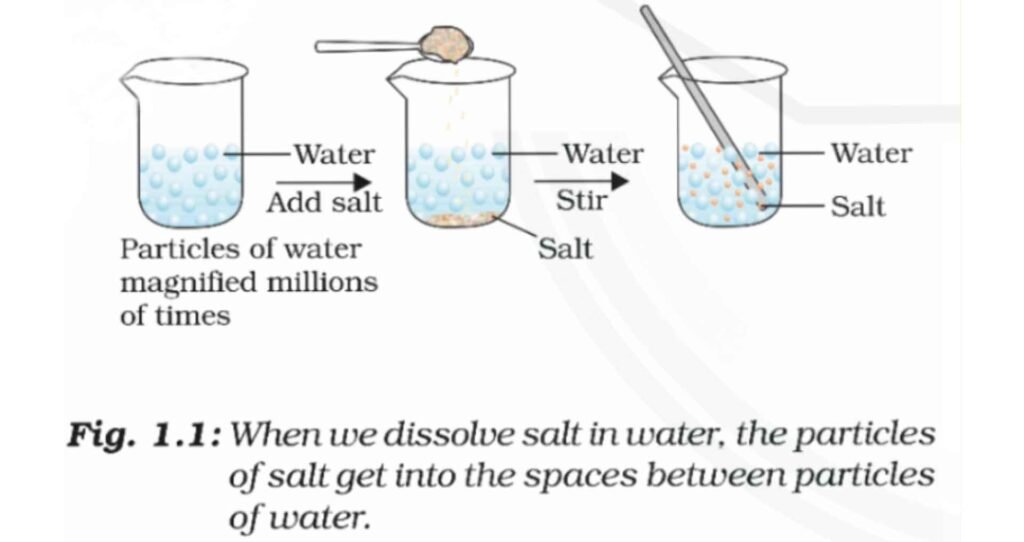
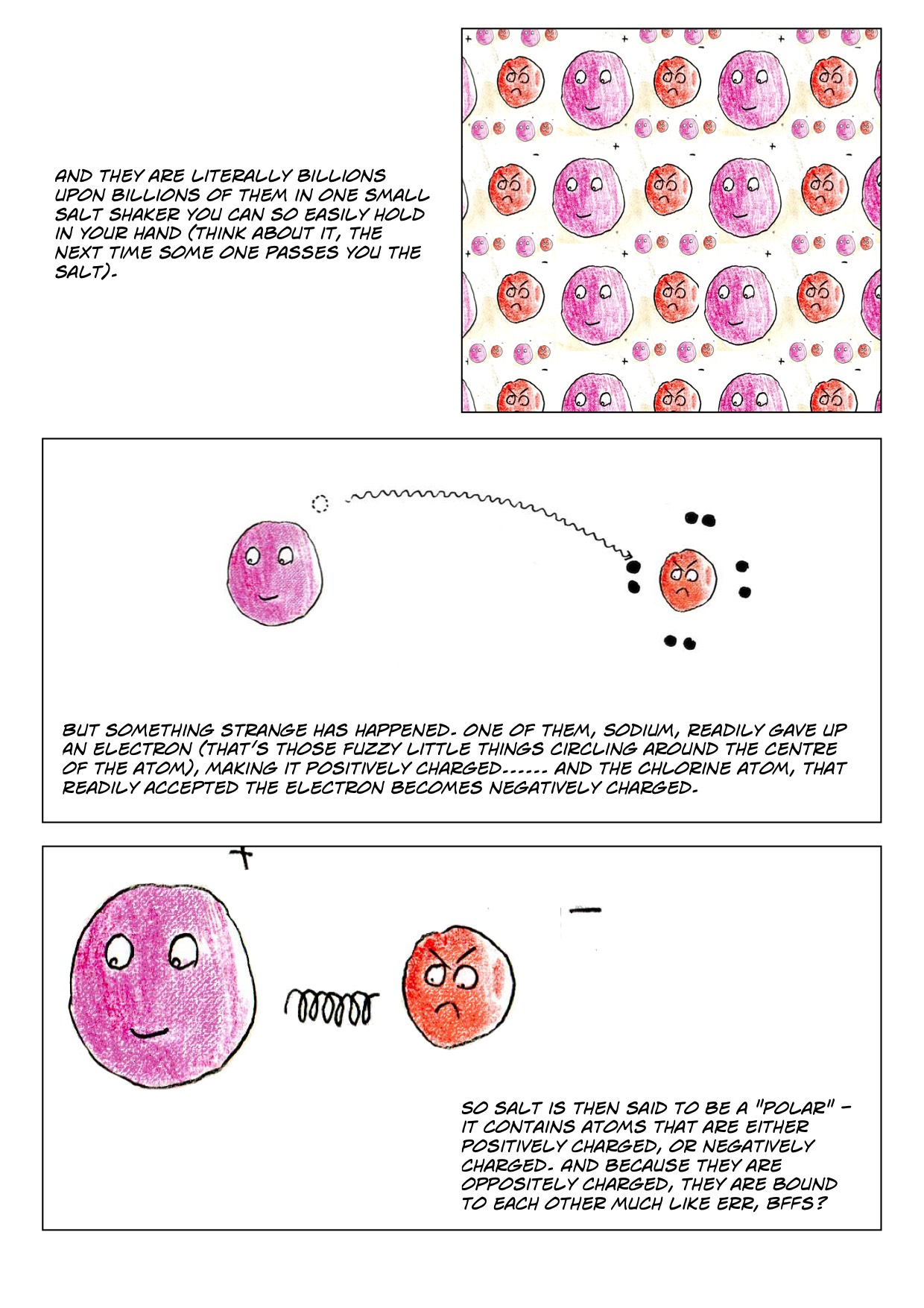
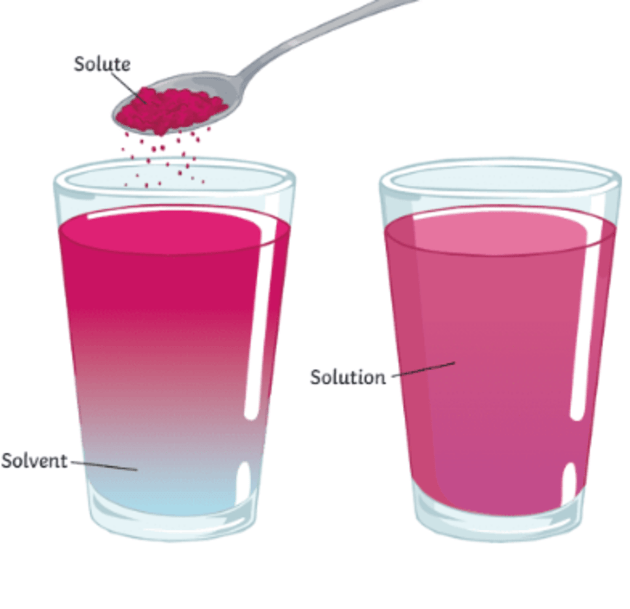
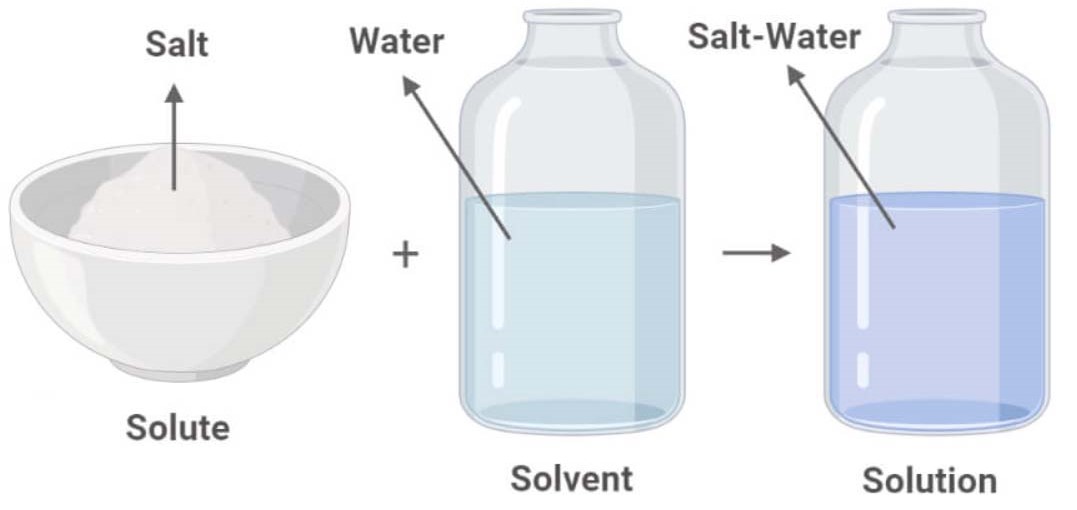
A Salt Will Dissolve In Water To Form
A Salt Will Dissolve In Water To Form - When dissolved in water, a. Substances that are ____ give up hydrogen ions when they dissolve in water. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. This process is called hydration,. A salt will dissolve in water to form. Salt dissolves in water due to the polar nature of both substances. Water is a polar molecule, which means it has a slightly. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Salt dissolved in water is a rough description of earth's oceans.
Substances that are ____ give up hydrogen ions when they dissolve in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. This process is called hydration,. In chemistry, it results in a solution, as the ionic bond of nacl. Water is a polar molecule, which means it has a slightly. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. When dissolved in water, a. Four of the five answers listed below are characteristics of water. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. A salt will dissolve in water to form.
In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Salt dissolves in water due to the polar nature of both substances. This process is called hydration,. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. A salt will dissolve in water to form. Substances that are ____ give up hydrogen ions when they dissolve in water. Water is a polar molecule, which means it has a slightly. When dissolved in water, a. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them.
How Does Salt Dissolve In Water Power Up Cook
This process is called hydration,. Substances that are ____ give up hydrogen ions when they dissolve in water. Water is a polar molecule, which means it has a slightly. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. Salt dissolves in water due to the polar nature of both.
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik
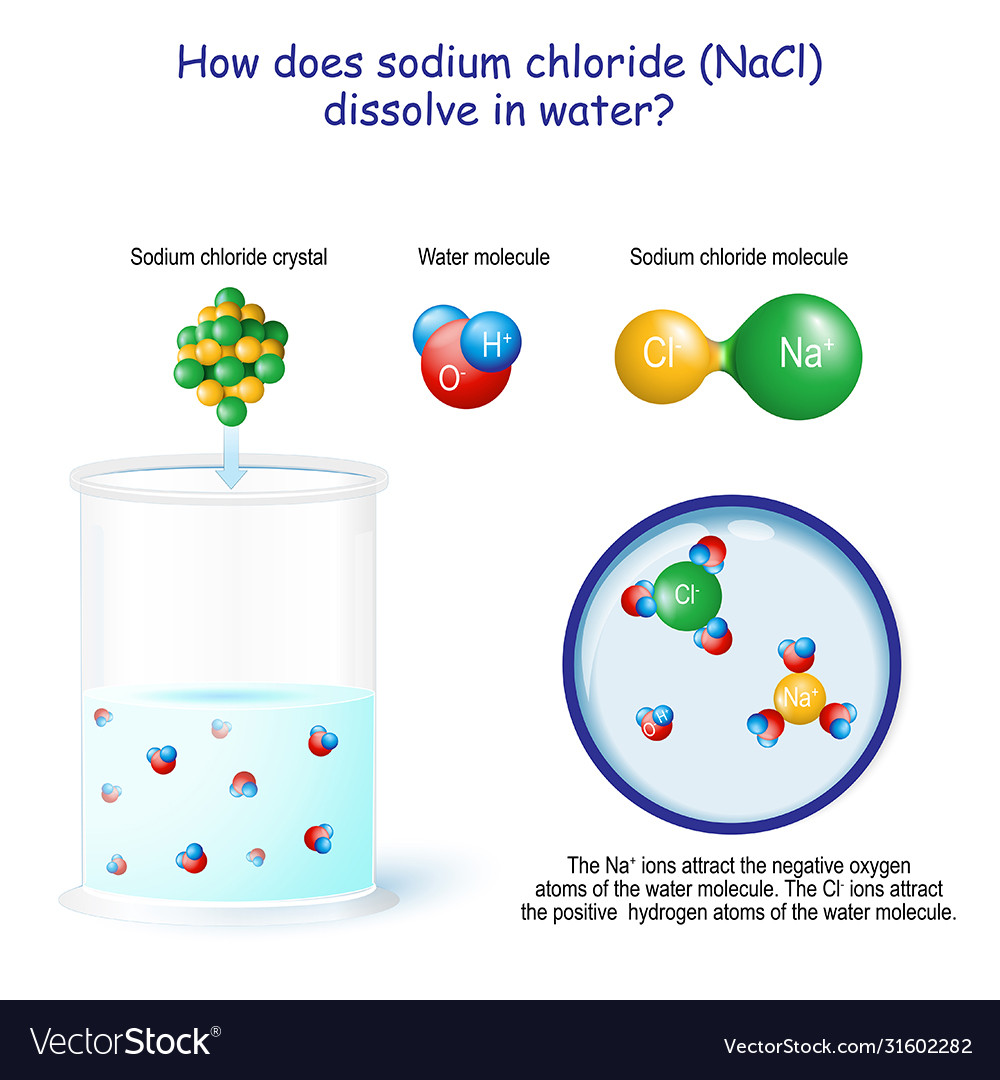
When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. This process is called hydration,. Substances that are ____ give up hydrogen ions when they dissolve in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt.
Why does salt dissolve in water? AQuriousMind
Substances that are ____ give up hydrogen ions when they dissolve in water. This process is called hydration,. When dissolved in water, a. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Four of the five answers listed below are characteristics of water.
What is Dissolving? Answered Twinkl Teaching Wiki
This process is called hydration,. Salt dissolves in water due to the polar nature of both substances. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Water is a polar molecule, which means it has a slightly. In chemistry, it results in a solution, as.
Is Dissolving Salt in Water a Chemical Change or a Physical Change
This process is called hydration,. Four of the five answers listed below are characteristics of water. Salt dissolved in water is a rough description of earth's oceans. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. At the molecular level, salt dissolves in water due to electrical charges and.
Solute Energy Education
When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. When dissolved in water, a. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Four of the five answers listed below are characteristics of water..
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt
When dissolved in water, a. Salt dissolved in water is a rough description of earth's oceans. This process is called hydration,. A salt will dissolve in water to form. Substances that are ____ give up hydrogen ions when they dissolve in water.
Why does salt dissolve in water? AQuriousMind
Water is a polar molecule, which means it has a slightly. Salt dissolved in water is a rough description of earth's oceans. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. When dissolved in water, a. Four of the five answers listed below are characteristics.
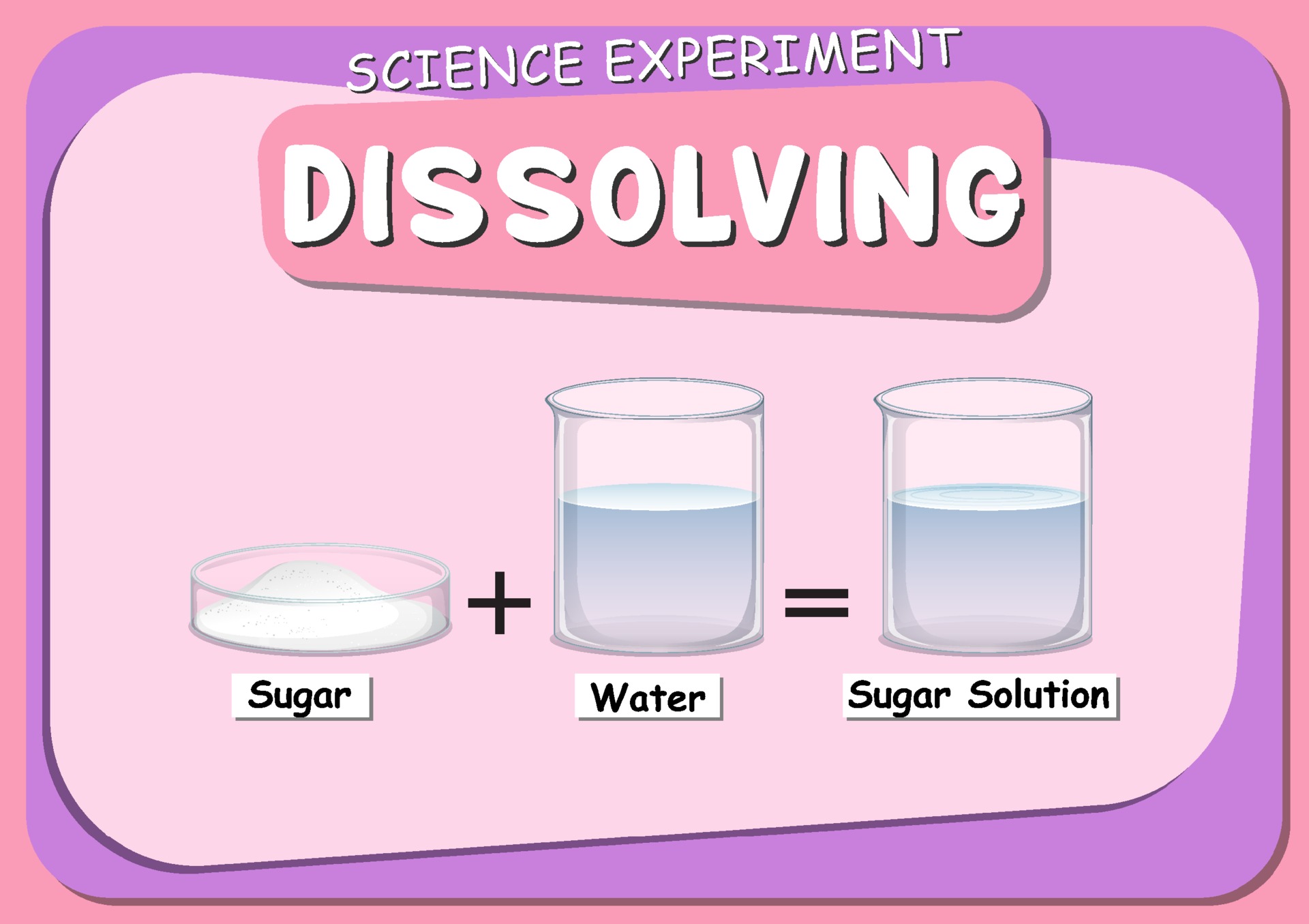
Dissolving science experiment with sugar dissolve in water 3333021
A salt will dissolve in water to form. Substances that are ____ give up hydrogen ions when they dissolve in water. Salt dissolved in water is a rough description of earth's oceans. Salt dissolves in water due to the polar nature of both substances. Water is a polar molecule, which means it has a slightly.
Ions In Aqueous Solution Infographic Diagram Showing, 44 OFF
When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. When dissolved in water, a. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Four of the five answers listed below are characteristics of water..
When Salt Is Added To Water, The Water Molecules Surround The Ions And Form A Sphere Of Hydration Around Them.
In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Salt dissolved in water is a rough description of earth's oceans. Four of the five answers listed below are characteristics of water.
This Process Is Called Hydration,.
Substances that are ____ give up hydrogen ions when they dissolve in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. A salt will dissolve in water to form. Salt dissolves in water due to the polar nature of both substances.
When Dissolved In Water, A.
Water is a polar molecule, which means it has a slightly.