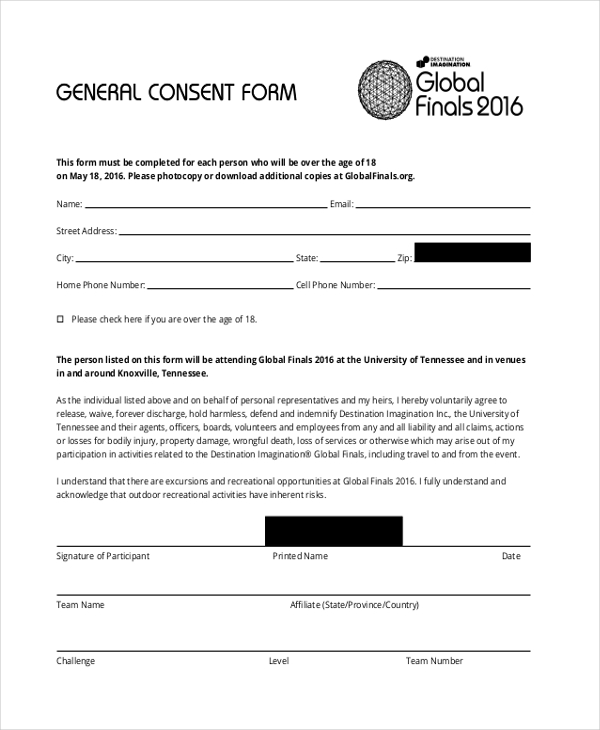
A General Requirement For The Informed Consent Form
A General Requirement For The Informed Consent Form - This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. (1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the.
(1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a.
This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. (1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in.
FREE 15+ Informed Consent Form Samples, PDF, MS Word, Google Docs, Excel
(1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. In.
General Informed Consent Form Printable Consent Form
This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. (1) before involving.
Creating a Professional Counseling Informed Consent Form
(1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent.
FREE 27+ Sample Consent Forms in PDF MS Word Excel
A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. (1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. In.
Free Research Informed Consent Form PDF Word eForms
A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. (1) before involving.
Informed consent in medical negligence claims Undergraduate Laws Blog
In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. (1) before involving.
A New Informed Consent Form PostGradDC
A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. (1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent.
FREE 45+ Consent Forms in PDF MS Word Excel
(1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. In.
Informed consent Data Management Expert Guide
This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development. (1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. In.
Informed Consent Form Template Word PDF Docs
(1) before involving a human subject in research covered by this policy, an investigator shall obtain the legally effective informed consent of the. In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent.
(1) Before Involving A Human Subject In Research Covered By This Policy, An Investigator Shall Obtain The Legally Effective Informed Consent Of The.
In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in. This document is structured to first present general guidance on fda’s regulatory requirements for informed consent and a. A) outline the requirements for obtaining adequate and legal informed consent from subjects participating in research and development.